Abstract
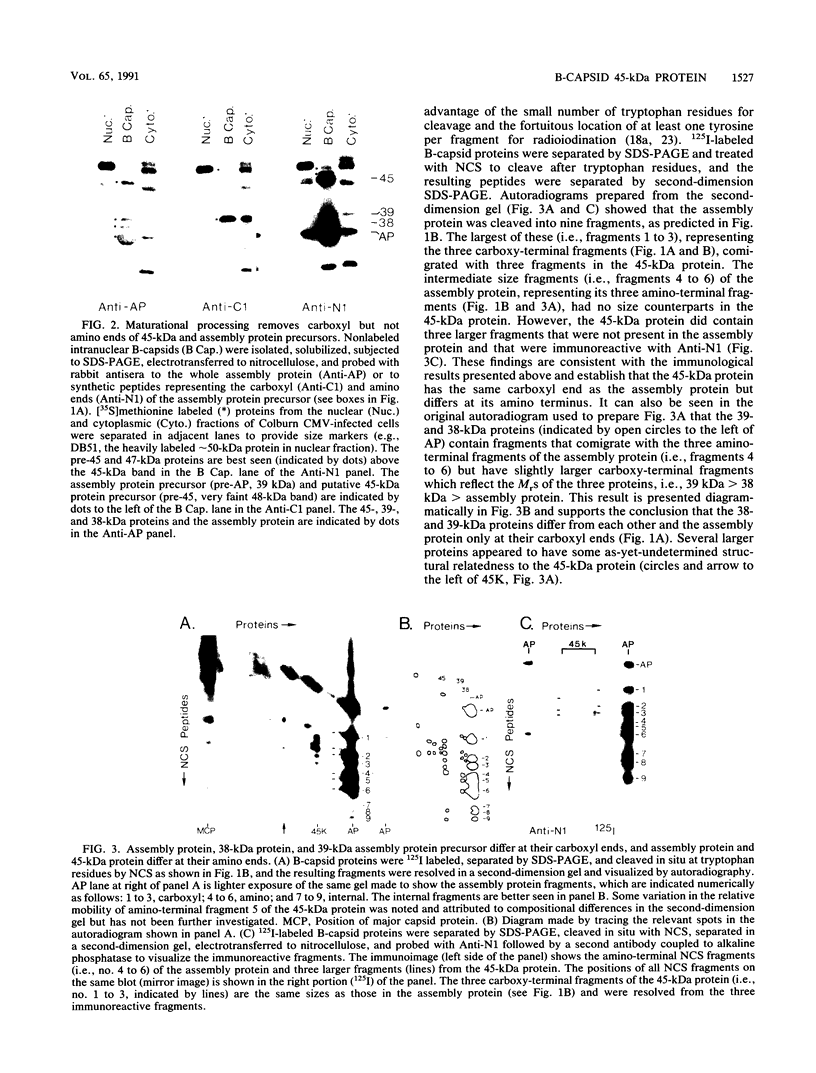
Intranuclear B-capsids from cytomegalovirus (strain Colburn)-infected cells contain an abundant 37-kDa assembly protein, thought to be involved in capsid formation, and three minor protein constituents (i.e., 45, 39, and 38 kDa) that are immunologically and structurally related to the assembly protein. In the experiments reported here, antisera produced against synthetic peptides were used in conjunction with chemical protein cleavage to examine the structural relationship of these proteins in more detail. Results of these experiments verify that the carboxyl end of the 39-kDa assembly protein precursor is lost during maturation and suggest that the 38-kDa protein may be a processing intermediate. It is shown that the 45-kDa protein is coterminal with the mature assembly protein at its carboxyl end but differs by a predicted 115-amino-acid extension at its amino terminus. In addition, evidence is presented that the 45-kDa protein has a 48-kDa precursor and a 47-kDa putative processing intermediate which have the same carboxy-terminal sequences and undergo the same maturational events as those of the assembly protein. A working model considering the structural relationship of these proteins is presented.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benko D. M., Haltiwanger R. S., Hart G. W., Gibson W. Virion basic phosphoprotein from human cytomegalovirus contains O-linked N-acetylglucosamine. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2573–2577. doi: 10.1073/pnas.85.8.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D. K., Roizman B., Pereira L. Characterization of post-translational products of herpes simplex virus gene 35 proteins binding to the surfaces of full capsids but not empty capsids. J Virol. 1984 Jan;49(1):142–153. doi: 10.1128/jvi.49.1.142-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Cox M. A., Leahy J., Hardwick J. M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990 Jan;64(1):313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs W. E., Grose C. Varicella-zoster virus p32/p36 complex is present in both the viral capsid and the nuclear matrix of the infected cell. J Virol. 1986 Jan;57(1):155–164. doi: 10.1128/jvi.57.1.155-164.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W., Hunter T., Cogen B., Eckhart W. Altered virion proteins of a temperature-sensitive mutant of polyoma virus, ts59. Virology. 1977 Jul 1;80(1):21–41. doi: 10.1016/0042-6822(77)90378-6. [DOI] [PubMed] [Google Scholar]
- Gibson W., Marcy A. I., Comolli J. C., Lee J. Identification of precursor to cytomegalovirus capsid assembly protein and evidence that processing results in loss of its carboxy-terminal end. J Virol. 1990 Mar;64(3):1241–1249. doi: 10.1128/jvi.64.3.1241-1249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983 Jul 30;128(2):391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- Gibson W. Structural and nonstructural proteins of strain Colburn cytomegalovirus. Virology. 1981 Jun;111(2):516–537. doi: 10.1016/0042-6822(81)90354-8. [DOI] [PubMed] [Google Scholar]
- Irmiere A., Gibson W. Isolation and characterization of a noninfectious virion-like particle released from cells infected with human strains of cytomegalovirus. Virology. 1983 Oct 15;130(1):118–133. doi: 10.1016/0042-6822(83)90122-8. [DOI] [PubMed] [Google Scholar]
- Irmiere A., Gibson W. Isolation of human cytomegalovirus intranuclear capsids, characterization of their protein constituents, and demonstration that the B-capsid assembly protein is also abundant in noninfectious enveloped particles. J Virol. 1985 Oct;56(1):277–283. doi: 10.1128/jvi.56.1.277-283.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson G. R., Schaffer M. H., Stark G. R., Vanaman T. C. Specific chemical cleavage in high yield at the amino peptide bonds of cysteine and cystine residues. J Biol Chem. 1973 Oct 10;248(19):6583–6591. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lee J. Y., Irmiere A., Gibson W. Primate cytomegalovirus assembly: evidence that DNA packaging occurs subsequent to B capsid assembly. Virology. 1988 Nov;167(1):87–96. doi: 10.1016/0042-6822(88)90057-8. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Ochs D. A new method for partial peptide mapping using N-chlorosuccinimide/urea and peptide silver staining in sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1982 Dec;127(2):453–457. doi: 10.1016/0003-2697(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B. The pre-S region of hepadnavirus envelope proteins. Adv Virus Res. 1988;34:65–142. doi: 10.1016/s0065-3527(08)60516-3. [DOI] [PubMed] [Google Scholar]
- Perdue M. L., Cohen J. C., Kemp M. C., Randall C. C., O'Callaghan D. J. Characterization of three species of nucleocapsids of equine herpesvirus type-1 (EHV-1). Virology. 1975 Mar;64(1):187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Preston V. G., Coates J. A., Rixon F. J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983 Mar;45(3):1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., Cross A. M., Addison C., Preston V. G. The products of herpes simplex virus type 1 gene UL26 which are involved in DNA packaging are strongly associated with empty but not with full capsids. J Gen Virol. 1988 Nov;69(Pt 11):2879–2891. doi: 10.1099/0022-1317-69-11-2879. [DOI] [PubMed] [Google Scholar]
- Robson L., Gibson W. Primate cytomegalovirus assembly protein: genome location and nucleotide sequence. J Virol. 1989 Feb;63(2):669–676. doi: 10.1128/jvi.63.2.669-676.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk P., Pietschmann S., Gelderblom H., Pauli G., Ludwig H., Schenck P. Monoclonal antibodies against herpes simplex virus type 1-infected nuclei defining and localizing the ICP8 protein, 65K DNA-binding protein and polypeptides of the ICP35 family. J Gen Virol. 1988 Jan;69(Pt 1):99–111. doi: 10.1099/0022-1317-69-1-99. [DOI] [PubMed] [Google Scholar]
- Shaw J. E., Murialdo H. Morphogenetic genes C and Nu3 overlap in bacteriophage lambda. Nature. 1980 Jan 3;283(5742):30–35. doi: 10.1038/283030a0. [DOI] [PubMed] [Google Scholar]
- Sherman G., Bachenheimer S. L. Characterization of intranuclear capsids made by ts morphogenic mutants of HSV-1. Virology. 1988 Apr;163(2):471–480. doi: 10.1016/0042-6822(88)90288-7. [DOI] [PubMed] [Google Scholar]
- Stark G. R. Cleavage at cysteine after cyanylation. Methods Enzymol. 1977;47:129–132. doi: 10.1016/0076-6879(77)47015-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]