Abstract
A characterization of the antigenic determinants (epitopes) of the glycoprotein (G) of infectious hematopoietic necrosis virus was made by expressing different regions of the G gene in Escherichia coli. A cDNA copy of the G gene was divided into four fragments by TaqI digestion, and the fragments were subcloned into pATH vectors, placing the expression of each G gene fragment under control of the trpE promoter. The resulting plasmids, pXL2, pXL3, and pXL7, encoded trpE-G fusion proteins subsequently detected with anti-infectious hematopoietic necrosis virus sera by Western immunoblots. A comparison of reactivities of the fusion proteins encoded by these plasmids was made by Western immunoblot and radioimmunoassay with a number of anti-G specific monoclonal antibodies (MAbs). The nonneutralizing MAb 136J reacted with the trpE-G fusion protein encoded by pXL3 and fusion proteins encoded by plasmids p52G and p618G, which were described in previous studies (R. D. Gilmore, Jr., H. M. Engelking, D. S. Manning, and J. C. Leong. Bio/Technology 6:295-300, 1988). Another nonneutralizing MAb, 2F, bound to the pXL3 fusion protein, and the neutralizing MAb RB/B5 recognized the pXL7 fusion protein. All fusion proteins were tested as vaccines in rainbow trout fry. Although significant protection was induced by all fusion proteins, the pXL3 fusion protein was most effective as a vaccine.
Full text
PDF
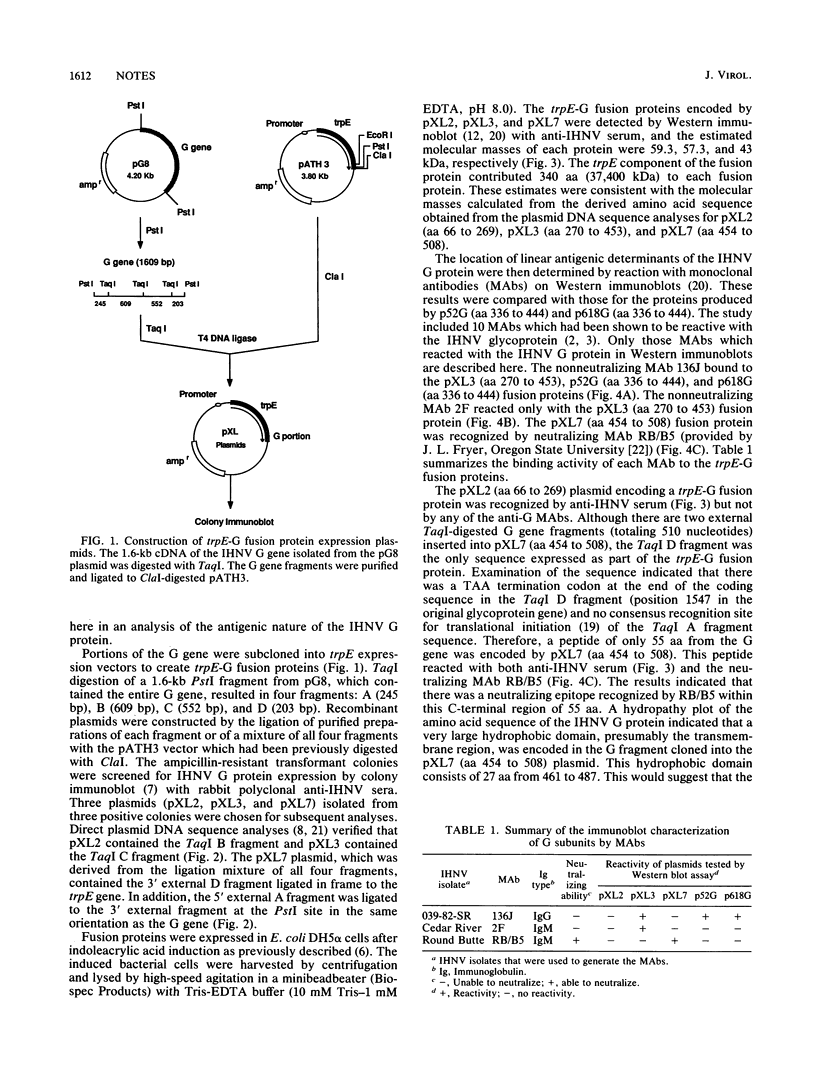


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dietzschold B., Wunner W. H., Wiktor T. J., Lopes A. D., Lafon M., Smith C. L., Koprowski H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci U S A. 1983 Jan;80(1):70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelking H. M., Leong J. C. The glycoprotein of infectious hematopoietic necrosis virus elicits neutralizing antibody and protective responses. Virus Res. 1989 Jul;13(3):213–230. doi: 10.1016/0168-1702(89)90017-8. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Hughes S. H. Use of antibodies to screen cDNA expression libraries prepared in plasmid vectors. Methods Enzymol. 1987;152:451–457. doi: 10.1016/0076-6879(87)52053-5. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Keil W., Wagner R. R. Epitope mapping by deletion mutants and chimeras of two vesicular stomatitis virus glycoprotein genes expressed by a vaccinia virus vector. Virology. 1989 Jun;170(2):392–407. doi: 10.1016/0042-6822(89)90430-3. [DOI] [PubMed] [Google Scholar]
- Koener J. F., Passavant C. W., Kurath G., Leong J. Nucleotide sequence of a cDNA clone carrying the glycoprotein gene of infectious hematopoietic necrosis virus, a fish rhabdovirus. J Virol. 1987 May;61(5):1342–1349. doi: 10.1128/jvi.61.5.1342-1349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurath G., Leong J. C. Characterization of infectious hematopoietic necrosis virus mRNA species reveals a nonvirion rhabdovirus protein. J Virol. 1985 Feb;53(2):462–468. doi: 10.1128/jvi.53.2.462-468.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Structural proteins of two salmonid rhabdoviruses. J Virol. 1975 Apr;15(4):733–738. doi: 10.1128/jvi.15.4.733-738.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain B. B., Fryer J. L., Pilcher K. S. Physicochemical properties of RNA of salmonid hematopoietic necrosis virus (Oregon strain). Proc Soc Exp Biol Med. 1974 Jun;146(2):630–634. doi: 10.3181/00379727-146-38161. [DOI] [PubMed] [Google Scholar]
- Pilcher K. S., Fryer J. L. The viral diseases of fish: a review through 1978. Part 1: Diseases of proven viral etiology. Crit Rev Microbiol. 1980;7(4):287–363. doi: 10.3109/10408418009077984. [DOI] [PubMed] [Google Scholar]
- Seif I., Coulon P., Rollin P. E., Flamand A. Rabies virulence: effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J Virol. 1985 Mar;53(3):926–934. doi: 10.1128/jvi.53.3.926-934.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. M., Weber D. K., Johnson T., Sakaguchi A. Y. Supercoil sequencing using unpurified templates produced by rapid boiling. Biotechniques. 1988 Oct;6(9):839, 841-3. [PubMed] [Google Scholar]