Abstract
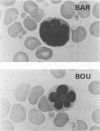
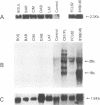
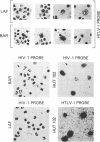
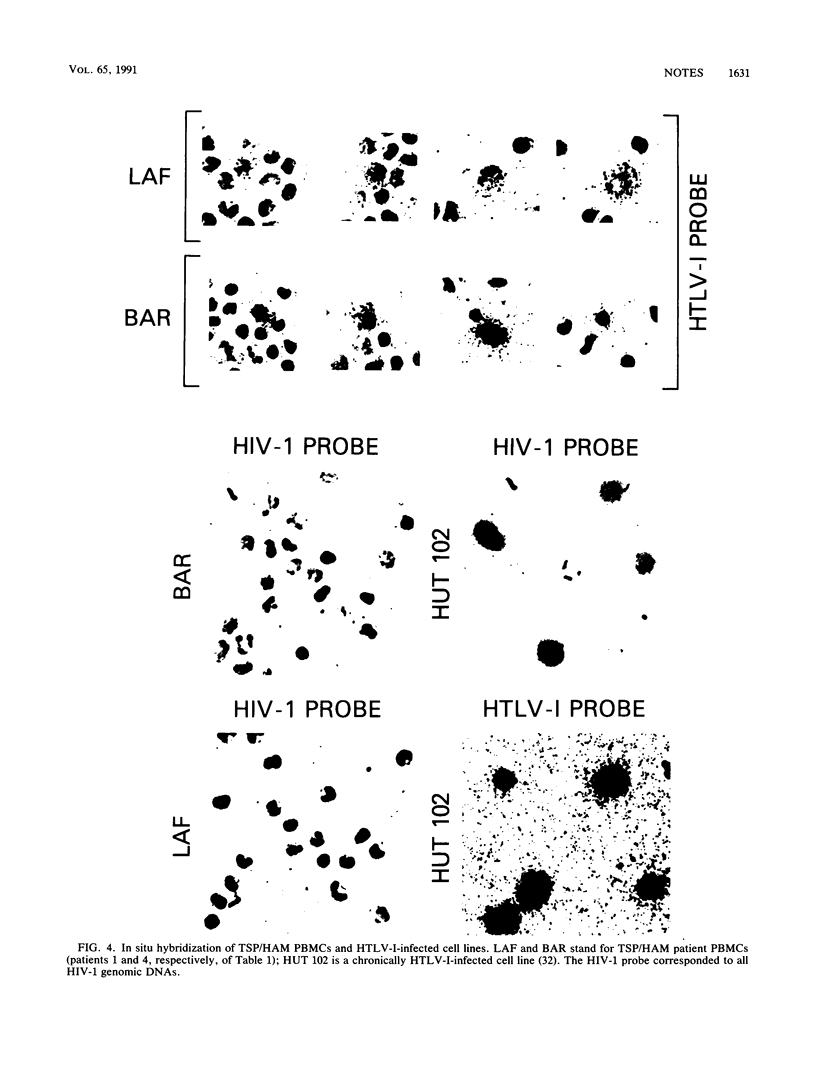
Tropical spastic paraparesis/human T-cell leukemia-lymphoma virus type I (HTLV-I)-associated myelopathy (TSP/HAM) is a chronic neurological illness epidemiologically associated with HTLV-I infection. We investigated the role of HTLV-I in the pathogenesis of this disease by studying viral expression in fresh uncultured peripheral blood mononuclear cells (PBMCs) of six patients of Caribbean origin with TSP/HAM. The PBMC genomic DNA of all the patients studied carried HTLV-I provirus, but viral expression was not detected by Northern (RNA) blot analysis of total cellular PBMC RNA. When the reverse transcriptase polymerase chain reaction technique was used with primers specific for the tax-rex mRNA, all of the samples were positive for this viral mRNA species, regardless of the duration of the illness (range, 2 to 13 years). The splice junctions for the tax-rex mRNA described in cases of HTLV-I-induced adult T-cell leukemia (position 5183 of the envelope and position 7302 of the pX region) were identical in three TSP/HAM cases studied. To ascertain whether viral expression occurred at a low level in many cells or at a high level in a few permissive cells, we performed in situ hybridization on fresh PBMCs from two patients (2 and 7 years after clinical diagnosis), seeking HTLV-I RNA sequences. Our finding indicated that in vivo HTLV-I expression occurred at a high level in a few cells (1 of every 5,000 PBMCs) in both cases studied. The fact that cells of all six patients with TSP/HAM were positive for viral expression, regardless of the time lag from diagnosis, suggests that persistent expression of a viral product(s) may be pivotal in the pathogenesis of TSP/HAM.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., De Rossi A., Feinberg M. B., Wong-Staal F., Franchini G. Molecular analysis of a deletion mutant provirus of type I human T-cell lymphotropic virus: evidence for a doubly spliced x-lor mRNA. Proc Natl Acad Sci U S A. 1986 Jan;83(1):38–42. doi: 10.1073/pnas.83.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard D. W., Böhnlein E., Lowenthal J. W., Wano Y., Franza B. R., Greene W. C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988 Sep 23;241(4873):1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- Blattner W. A., Kalyanaraman V. S., Robert-Guroff M., Lister T. A., Galton D. A., Sarin P. S., Crawford M. H., Catovsky D., Greaves M., Gallo R. C. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int J Cancer. 1982 Sep 15;30(3):257–264. doi: 10.1002/ijc.2910300302. [DOI] [PubMed] [Google Scholar]
- Clarke M. F., Gelmann E. P., Reitz M. S., Jr Homology of human T-cell leukaemia virus envelope gene with class I HLA gene. Nature. 1983 Sep 1;305(5929):60–62. doi: 10.1038/305060a0. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Feinberg M. B., Wolf J. B., Holbrook N. J., Wong-Staal F., Leonard W. J. Regulation of the human interleukin-2 receptor alpha chain promoter: activation of a nonfunctional promoter by the transactivator gene of HTLV-I. Cell. 1987 Apr 10;49(1):47–56. doi: 10.1016/0092-8674(87)90754-9. [DOI] [PubMed] [Google Scholar]
- Cruickshank J. K., Rudge P., Dalgleish A. G., Newton M., McLean B. N., Barnard R. O., Kendall B. E., Miller D. H. Tropical spastic paraparesis and human T cell lymphotropic virus type 1 in the United Kingdom. Brain. 1989 Aug;112(Pt 4):1057–1090. doi: 10.1093/brain/112.4.1057. [DOI] [PubMed] [Google Scholar]
- Dalgleish A., Richardson J., Matutes E., Cruickshank K., Newell A., Sinclair A., Thorpe R., Brasher M., Weber J., Catovsky D. HTLV-1 infection in tropical spastic paraparesis: lymphocyte culture and serologic response. AIDS Res Hum Retroviruses. 1988 Dec;4(6):475–485. doi: 10.1089/aid.1988.4.475. [DOI] [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Paskalis H., Kleinman-Ewing C., Wong-Staal F., Pavlakis G. N. The pX protein of HTLV-I is a transcriptional activator of its long terminal repeats. Science. 1985 Aug 16;229(4714):675–679. doi: 10.1126/science.2992082. [DOI] [PubMed] [Google Scholar]
- Fleming A. F., Maharajan R., Abraham M., Kulkarni A. G., Bhusnurmath S. R., Okpara R. A., Williams E., Akinsete I., Schneider J., Bayer H. Antibodies to HTLV-I in Nigerian blood-donors, their relatives and patients with leukaemias, lymphomas and other diseases. Int J Cancer. 1986 Dec 15;38(6):809–813. doi: 10.1002/ijc.2910380605. [DOI] [PubMed] [Google Scholar]
- Franchini G., Wong-Staal F., Gallo R. C. Human T-cell leukemia virus (HTLV-I) transcripts in fresh and cultured cells of patients with adult T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6207–6211. doi: 10.1073/pnas.81.19.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Gessain A., Caudie C., Gout O., Vernant J. C., Maurs L., Giordano C., Malone G., Tournier-Lasserve E., Essex M., de-Thé G. Intrathecal synthesis of antibodies to human T lymphotropic virus type I and the presence of IgG oligoclonal bands in the cerebrospinal fluid of patients with endemic tropical spastic paraparesis. J Infect Dis. 1988 Jun;157(6):1226–1234. doi: 10.1093/infdis/157.6.1226. [DOI] [PubMed] [Google Scholar]
- Gessain A., Saal F., Gout O., Daniel M. T., Flandrin G., de The G., Peries J., Sigaux F. High human T-cell lymphotropic virus type I proviral DNA load with polyclonal integration in peripheral blood mononuclear cells of French West Indian, Guianese, and African patients with tropical spastic paraparesis. Blood. 1990 Jan 15;75(2):428–433. [PubMed] [Google Scholar]
- Gout O., Baulac M., Gessain A., Semah F., Saal F., Périès J., Cabrol C., Foucault-Fretz C., Laplane D., Sigaux F. Rapid development of myelopathy after HTLV-I infection acquired by transfusion during cardiac transplantation. N Engl J Med. 1990 Feb 8;322(6):383–388. doi: 10.1056/NEJM199002083220607. [DOI] [PubMed] [Google Scholar]
- Gout O., Gessain A., Bolgert F., Saal F., Tournier-Lasserve E., Lasneret J., Caudie C., Brunet P., De-Thé G., Lhermitte F. Chronic myelopathies associated with human T-lymphotropic virus type I. A clinical, serologic, and immunovirologic study of ten patients in France. Arch Neurol. 1989 Mar;46(3):255–260. doi: 10.1001/archneur.1989.00520390021009. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Marselle L. M., Gallo R. C., Wong-Staal F. Detection of lymphocytes expressing human T-lymphotropic virus type III in lymph nodes and peripheral blood from infected individuals by in situ hybridization. Proc Natl Acad Sci U S A. 1986 Feb;83(3):772–776. doi: 10.1073/pnas.83.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino H., Shimoyama M., Miwa M., Sugimura T. Detection of lymphocytes producing a human retrovirus associated with adult T-cell leukemia by syncytia induction assay. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7337–7341. doi: 10.1073/pnas.80.23.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi S., Eiraku N., Osame M., Izumo S., Kubota R., Maruyama I., Matsumoto M., Niimura T., Sonoda S. Activated T lymphocytes in cerebrospinal fluid of patients with HTLV-I-associated myelopathy (HAM/TSP). J Neuroimmunol. 1989 Dec;25(2-3):251–254. doi: 10.1016/0165-5728(89)90143-4. [DOI] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson S., Zaninovic V., Mora C., Rodgers-Johnson P., Sheremata W. A., Gibbs C. J., Jr, Gajdusek C., McFarlin D. E. Immunological findings in neurological diseases associated with antibodies to HTLV-I: activated lymphocytes in tropical spastic paraparesis. Ann Neurol. 1988;23 (Suppl):S196–S200. doi: 10.1002/ana.410230744. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Nakao Y., Ito Y., Aoki T., Gallo R. C. Natural antibodies to the structural core protein (p24) of the human T-cell leukemia (lymphoma) retrovirus found in sera of leukemia patients in Japan. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1653–1657. doi: 10.1073/pnas.79.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki E. S., Clark S. S., Coyne M. Y., Smith S. D., Champlin R., Witte O. N., McCormick F. P. Diagnosis of chronic myeloid and acute lymphocytic leukemias by detection of leukemia-specific mRNA sequences amplified in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5698–5702. doi: 10.1073/pnas.85.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Shimoyama M., Tobinai K., Ito M., Ito S., Ikeda S., Tajima K., Shimotohno K., Sugimura T. Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5620–5624. doi: 10.1073/pnas.86.14.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama M., Shibuya H., Harada H., Hatakeyama M., Seiki M., Fujita T., Inoue J., Yoshida M., Taniguchi T. Evidence for aberrant activation of the interleukin-2 autocrine loop by HTLV-1-encoded p40x and T3/Ti complex triggering. Cell. 1987 Jan 30;48(2):343–350. doi: 10.1016/0092-8674(87)90437-5. [DOI] [PubMed] [Google Scholar]
- Minato S., Itoyama Y., Fujii N., Kira J., Goto I., Yamamoto N. Activated T cells in HTLV-I-associated myelopathy: autologous mixed lymphocyte reaction. Ann Neurol. 1989 Sep;26(3):398–401. doi: 10.1002/ana.410260316. [DOI] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Nerenberg M., Hinrichs S. H., Reynolds R. K., Khoury G., Jay G. The tat gene of human T-lymphotropic virus type 1 induces mesenchymal tumors in transgenic mice. Science. 1987 Sep 11;237(4820):1324–1329. doi: 10.1126/science.2888190. [DOI] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986 May 3;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert-Guroff M., Nakao Y., Notake K., Ito Y., Sliski A., Gallo R. C. Natural antibodies to human retrovirus HTLV in a cluster of Japanese patients with adult T cell leukemia. Science. 1982 Feb 19;215(4535):975–978. doi: 10.1126/science.6760397. [DOI] [PubMed] [Google Scholar]
- Rodgers-Johnson P., Gajdusek D. C., Morgan O. S., Zaninovic V., Sarin P. S., Graham D. S. HTLV-I and HTLV-III antibodies and tropical spastic paraparesis. Lancet. 1985 Nov 30;2(8466):1247–1248. doi: 10.1016/s0140-6736(85)90778-0. [DOI] [PubMed] [Google Scholar]
- Sakai Y., Yao H., Sadoshima S., Fujishima M., Okochi K. Development of HTLV-I associated myelopathy (HAM) in a seroconverted patient for antibody to HTLV-I. J Neurol Neurosurg Psychiatry. 1989 Dec;52(12):1445–1445. doi: 10.1136/jnnp.52.12.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hikikoshi A., Taniguchi T., Yoshida M. Expression of the pX gene of HTLV-I: general splicing mechanism in the HTLV family. Science. 1985 Jun 28;228(4707):1532–1534. doi: 10.1126/science.2990031. [DOI] [PubMed] [Google Scholar]
- Siekevitz M., Feinberg M. B., Holbrook N., Wong-Staal F., Greene W. C. Activation of interleukin 2 and interleukin 2 receptor (Tac) promoter expression by the trans-activator (tat) gene product of human T-cell leukemia virus, type I. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5389–5393. doi: 10.1073/pnas.84.15.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamura K., Fujii M., Kannagi M., Sakitani M., Takeuchi M., Hinuma Y. Cell surface phenotypes and expression of viral antigens of various human cell lines carrying human T-cell leukemia virus. Int J Cancer. 1984 Aug 15;34(2):221–228. doi: 10.1002/ijc.2910340213. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Wong-Staal F., Hahn B., Manzari V., Colombini S., Franchini G., Gelmann E. P., Gallo R. C. A survey of human leukaemias for sequences of a human retrovirus. Nature. 1983 Apr 14;302(5909):626–628. doi: 10.1038/302626a0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M., Osame M., Kawai H., Toita M., Kuwasaki N., Nishida Y., Hiraki Y., Takahashi K., Nomura K., Sonoda S. Increased replication of HTLV-I in HTLV-I-associated myelopathy. Ann Neurol. 1989 Sep;26(3):331–335. doi: 10.1002/ana.410260304. [DOI] [PubMed] [Google Scholar]