Abstract
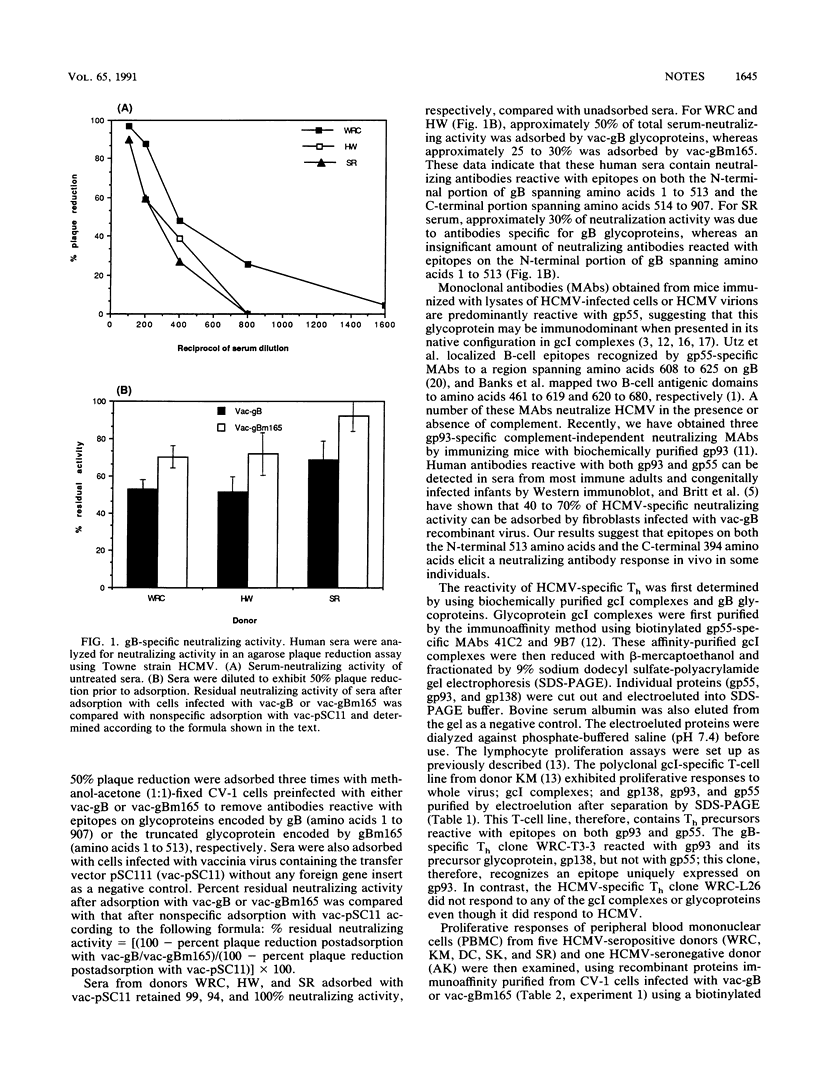
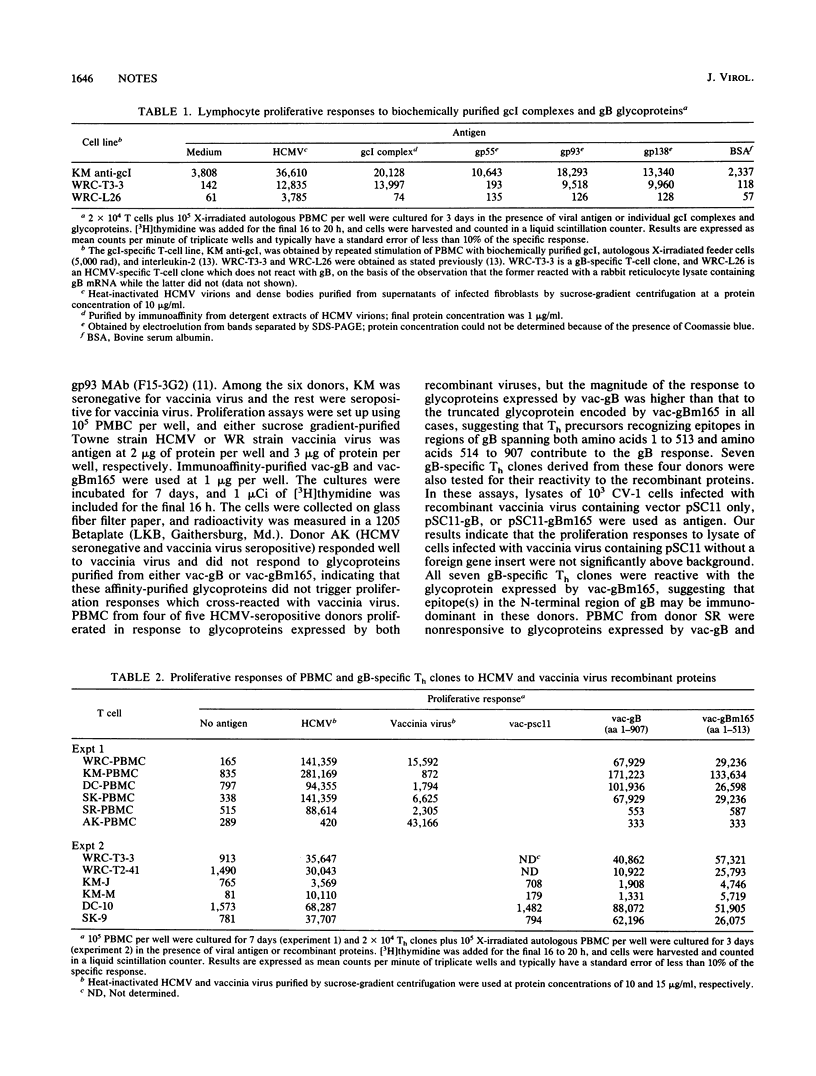
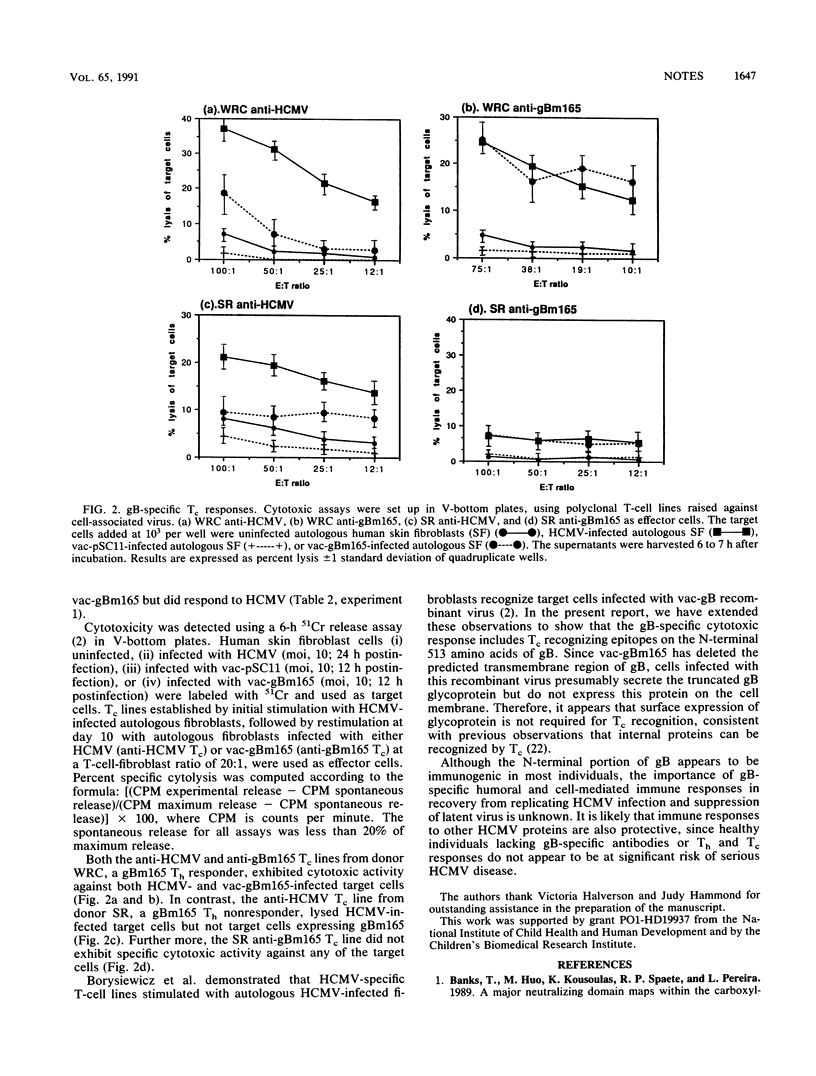
Host defense against human cytomegalovirus (HCMV) involves both humoral and cell-mediated immunity. In this report, human immune responses to glycoproteins encoded by the HCMV gB homolog gene have been examined by using glycoproteins purified by immunoaffinity from HCMV virions and recombinant proteins expressed by vaccinia viruses containing either the entire gB open reading frame or a C-terminal deletion mutant, gBm165, coding for the N-terminal 513 amino acids of gB. Neutralizing antibodies, helper T cells, and cytotoxic T cells reactive with epitopes on the N-terminal portion of gB were detected in some seropositive individuals, suggesting that this region of gB may be important in eliciting protective immunity during natural infection for some individuals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borysiewicz L. K., Hickling J. K., Graham S., Sinclair J., Cranage M. P., Smith G. L., Sissons J. G. Human cytomegalovirus-specific cytotoxic T cells. Relative frequency of stage-specific CTL recognizing the 72-kD immediate early protein and glycoprotein B expressed by recombinant vaccinia viruses. J Exp Med. 1988 Sep 1;168(3):919–931. doi: 10.1084/jem.168.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J., Auger D. Synthesis and processing of the envelope gp55-116 complex of human cytomegalovirus. J Virol. 1986 Apr;58(1):185–191. doi: 10.1128/jvi.58.1.185-191.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britt W. J. Neutralizing antibodies detect a disulfide-linked glycoprotein complex within the envelope of human cytomegalovirus. Virology. 1984 Jun;135(2):369–378. doi: 10.1016/0042-6822(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Britt W. J., Vugler L., Butfiloski E. J., Stephens E. B. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J Virol. 1990 Mar;64(3):1079–1085. doi: 10.1128/jvi.64.3.1079-1085.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretch D. R., Gehrz R. C., Stinski M. F. Characterization of a human cytomegalovirus glycoprotein complex (gcI). J Gen Virol. 1988 Jun;69(Pt 6):1205–1215. doi: 10.1099/0022-1317-69-6-1205. [DOI] [PubMed] [Google Scholar]
- Gretch D. R., Kari B., Rasmussen L., Gehrz R. C., Stinski M. F. Identification and characterization of three distinct families of glycoprotein complexes in the envelopes of human cytomegalovirus. J Virol. 1988 Mar;62(3):875–881. doi: 10.1128/jvi.62.3.875-881.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari B., Liu Y. N., Goertz R., Lussenhop N., Stinski M. F., Gehrz R. Structure and composition of a family of human cytomegalovirus glycoprotein complexes designated gC-I (gB). J Gen Virol. 1990 Nov;71(Pt 11):2673–2680. doi: 10.1099/0022-1317-71-11-2673. [DOI] [PubMed] [Google Scholar]
- Kari B., Lussenhop N., Goertz R., Wabuke-Bunoti M., Radeke R., Gehrz R. Characterization of monoclonal antibodies reactive to several biochemically distinct human cytomegalovirus glycoprotein complexes. J Virol. 1986 Nov;60(2):345–352. doi: 10.1128/jvi.60.2.345-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. N., Eckhardt J., Kari B., Gehrz R. C. General characterization of human cytomegalovirus-specific proliferative CD4+ T cell clones. Int J Cell Cloning. 1988 Sep;6(5):352–364. doi: 10.1002/stem.5530060506. [DOI] [PubMed] [Google Scholar]
- Mach M., Utz U., Fleckenstein B. Mapping of the major glycoprotein gene of human cytomegalovirus. J Gen Virol. 1986 Jul;67(Pt 7):1461–1467. doi: 10.1099/0022-1317-67-7-1461. [DOI] [PubMed] [Google Scholar]
- Pereira L., Hoffman M., Tatsuno M., Dondero D. Polymorphism of human cytomegalovirus glycoproteins characterized by monoclonal antibodies. Virology. 1984 Nov;139(1):73–86. doi: 10.1016/0042-6822(84)90331-3. [DOI] [PubMed] [Google Scholar]
- Rasmussen L., Nelson M., Neff M., Merigan T. C., Jr Characterization of two different human cytomegalovirus glycoproteins which are targets for virus neutralizing antibody. Virology. 1988 Apr;163(2):308–318. doi: 10.1016/0042-6822(88)90271-1. [DOI] [PubMed] [Google Scholar]
- Smiley B. L., Lupski J. R., Svec P. S., McMacken R., Godson G. N. Sequences of the Escherichia coli dnaG primase gene and regulation of its expression. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4550–4554. doi: 10.1073/pnas.79.15.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Thayer R. M., Probert W. S., Masiarz F. R., Chamberlain S. H., Rasmussen L., Merigan T. C., Pachl C. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology. 1988 Nov;167(1):207–225. doi: 10.1016/0042-6822(88)90071-2. [DOI] [PubMed] [Google Scholar]
- Utz U., Britt W., Vugler L., Mach M. Identification of a neutralizing epitope on glycoprotein gp58 of human cytomegalovirus. J Virol. 1989 May;63(5):1995–2001. doi: 10.1128/jvi.63.5.1995-2001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber P. C., Levine M., Glorioso J. C. Rapid identification of nonessential genes of herpes simplex virus type 1 by Tn5 mutagenesis. Science. 1987 May 1;236(4801):576–579. doi: 10.1126/science.3033824. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J., Lupski J. R. The use of transposon Tn5 mutagenesis in the rapid generation of correlated physical and genetic maps of DNA segments cloned into multicopy plasmids--a review. Gene. 1984 Feb;27(2):131–149. doi: 10.1016/0378-1119(84)90135-5. [DOI] [PubMed] [Google Scholar]



