Abstract
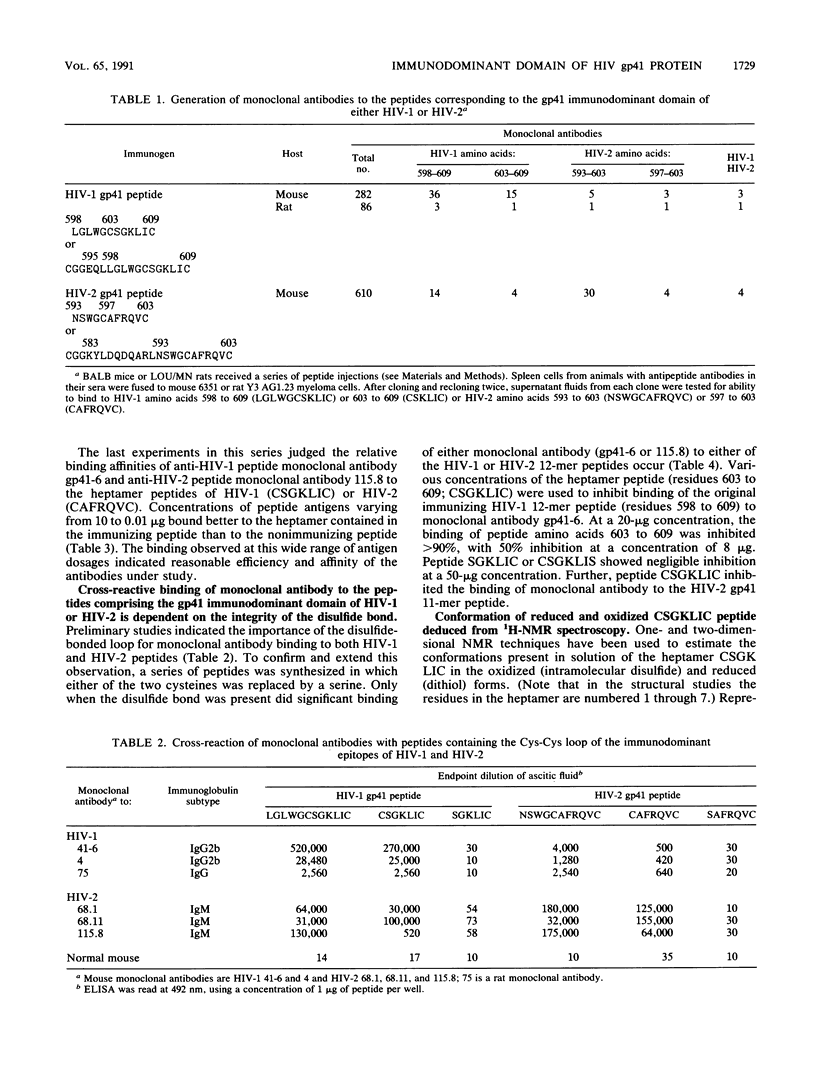
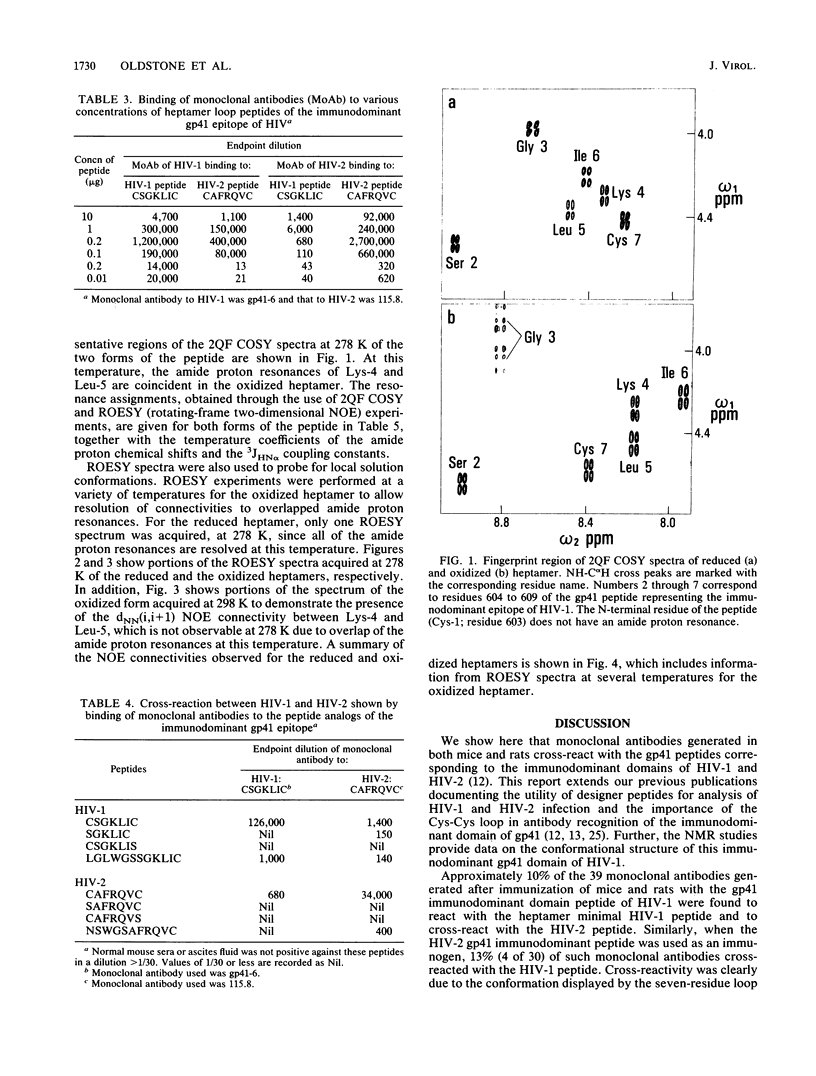
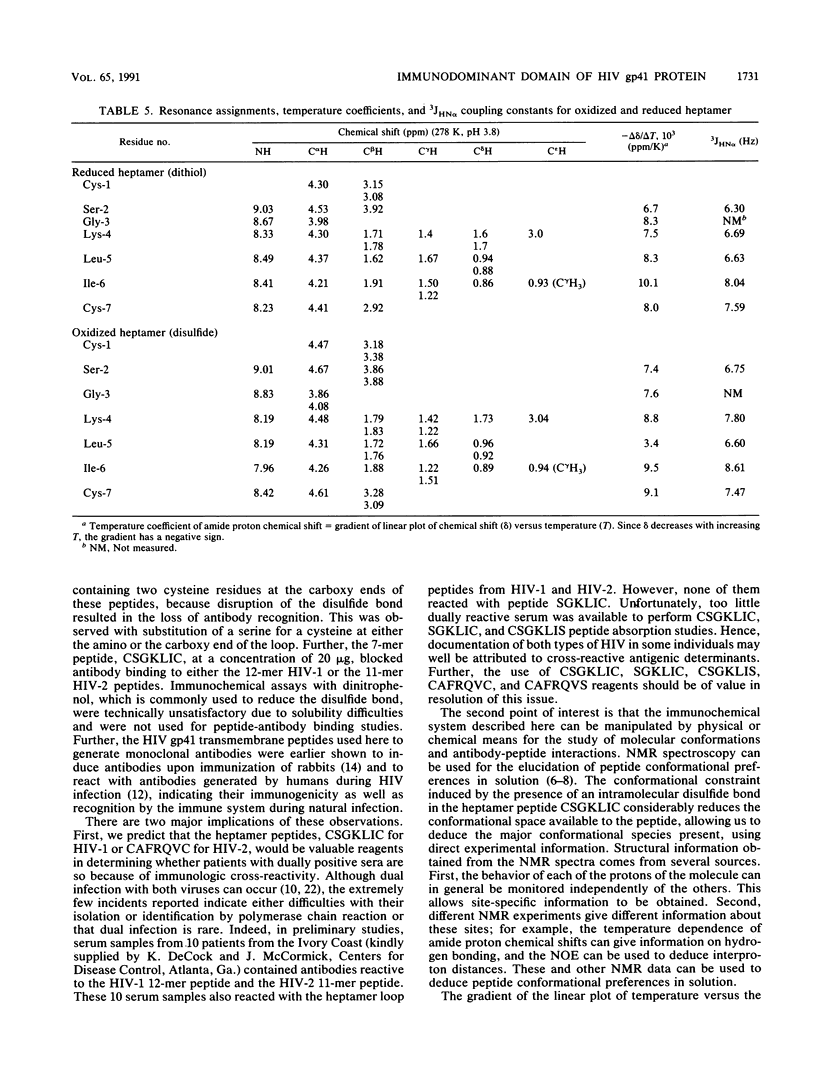
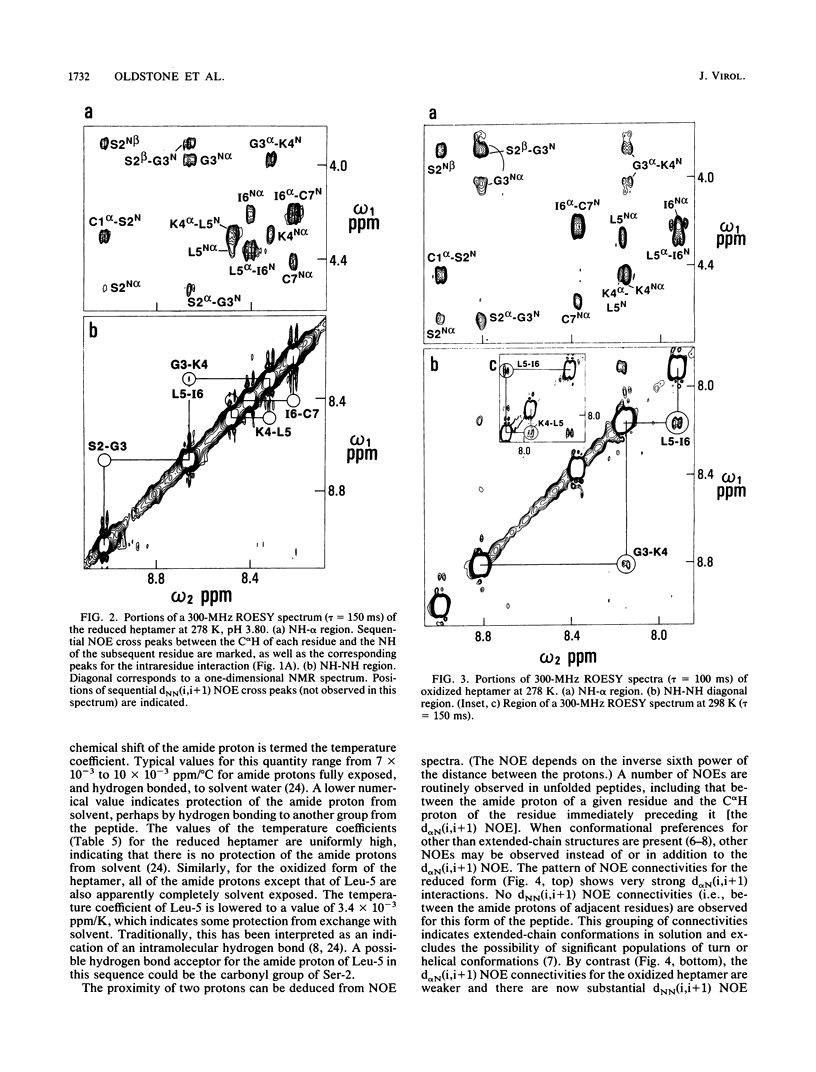
Thirty-six monoclonal antibodies from mice and three from rats were raised against a peptide corresponding to the immunodominant domain of the transmembrane gp41 protein of human immunodeficiency virus (HIV) type 1 (LGLWGCSGKLIC; amino acid residues 598 to 609). Of these, three monoclonal antibodies from the mice and one from a rat also reacted with the corresponding peptide derived from the HIV type 2 transmembrane gp41 protein (amino acid residues 593 to 603; NSWGCAFRQVC). Immunochemical studies using a variety of synthetic peptides indicated that the cross-reactivity was due to antibody binding to CSGKLIC of HIV type 1 or CAFRQVC of HIV type 2. Single amino acid substitutions for a cysteine at either the amino or the carboxy end of the peptide interrupted antibody binding, indicating that the site recognized was the Cys-XXXXX-Cys loop. Similar results were obtained when the 11-mer HIV type 2 gp41 peptide (amino acids 593 to 603) was inoculated into mice to raise monoclonal antibodies. In this instance, of 30 monoclonal antibodies developed, 4 reacted with both HIV type 1 and HIV type 2 peptides. The conformation of a seven-residue peptide, CSGKLIC, corresponding to residues 603 to 609 of the gp41 immunodominant epitope of HIV-1 was investigated by proton nuclear magnetic resonance spectroscopy. The immunologically active form of CSGKLIC contains an intramolecular disulfide bond and maintains a preference for a folded conformation, apparently including a type I reverse turn about the residues SGKL. No such preference is observed for the reduced form of the peptide, which contains two thiol groups. The presence of the disulfide bond is thus integral to the formation of the structure of the loop in solution. In agreement with this finding, elimination of the possibility of loop formation by substitution of S for C at the amino or carboxy termini of the 7-mer is accompanied by the failure of antibody binding to this peptide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosselman R. A., van Straaten F., Van Beveren C., Verma I. M., Vogt M. Analysis of the env gene of a molecularly cloned and biologically active Moloney mink cell focus-forming proviral DNA. J Virol. 1982 Oct;44(1):19–31. doi: 10.1128/jvi.44.1.19-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Lewicki H. A., Tomori O., Oldstone M. B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981 Aug;113(1):73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Cot M. C., Poulain M., Delagneau J. F., Peeters M., Brun-Vezinet F. Dual HIV-1 and HIV-2 infection in West Africa supported by synthetic peptide analysis. AIDS Res Hum Retroviruses. 1988 Aug;4(4):239–241. doi: 10.1089/aid.1988.4.239. [DOI] [PubMed] [Google Scholar]
- Dyrberg T., Oldstone M. B. Peptides as antigens. Importance of orientation. J Exp Med. 1986 Oct 1;164(4):1344–1349. doi: 10.1084/jem.164.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson H. J., Cross K. J., Houghten R. A., Wilson I. A., Wright P. E., Lerner R. A. The immunodominant site of a synthetic immunogen has a conformational preference in water for a type-II reverse turn. Nature. 1985 Dec 5;318(6045):480–483. doi: 10.1038/318480a0. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Rance M., Houghten R. A., Lerner R. A., Wright P. E. Folding of immunogenic peptide fragments of proteins in water solution. I. Sequence requirements for the formation of a reverse turn. J Mol Biol. 1988 May 5;201(1):161–200. doi: 10.1016/0022-2836(88)90446-9. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Rance M., Houghten R. A., Wright P. E., Lerner R. A. Folding of immunogenic peptide fragments of proteins in water solution. II. The nascent helix. J Mol Biol. 1988 May 5;201(1):201–217. doi: 10.1016/0022-2836(88)90447-0. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. A., Moreau J., Odehouri K., Seto D., Thomson-Honnebier G., Legg H., Barboza A., Cheng-Mayer C., Levy J. A. Simultaneous isolation of HIV-1 and HIV-2 from an AIDS patient. Lancet. 1988 Dec 17;2(8625):1389–1391. doi: 10.1016/s0140-6736(88)90586-7. [DOI] [PubMed] [Google Scholar]
- Foucault C., Lopez O., Jourdan G., Fournel J. J., Perret P., Gluckman J. C. Double HIV-1 and HIV-2 seropositivity among blood donors. Lancet. 1987 Jul 18;2(8551):165–166. doi: 10.1016/s0140-6736(87)92372-5. [DOI] [PubMed] [Google Scholar]
- Gnann J. W., Jr, McCormick J. B., Mitchell S., Nelson J. A., Oldstone M. B. Synthetic peptide immunoassay distinguishes HIV type 1 and HIV type 2 infections. Science. 1987 Sep 11;237(4820):1346–1349. doi: 10.1126/science.2888192. [DOI] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Nelson J. A., Oldstone M. B. Fine mapping of an immunodominant domain in the transmembrane glycoprotein of human immunodeficiency virus. J Virol. 1987 Aug;61(8):2639–2641. doi: 10.1128/jvi.61.8.2639-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Schwimmbeck P. L., Nelson J. A., Truax A. B., Oldstone M. B. Diagnosis of AIDS by using a 12-amino acid peptide representing an immunodominant epitope of the human immunodeficiency virus. J Infect Dis. 1987 Aug;156(2):261–267. doi: 10.1093/infdis/156.2.261. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Zinnecker M., Hamaoka T., Katz D. H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979 Feb 20;18(4):690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Norrby E., Biberfeld G., Chiodi F., von Gegerfeldt A., Nauclér A., Parks E., Lerner R. Discrimination between antibodies to HIV and to related retroviruses using site-directed serology. Nature. 1987 Sep 17;329(6136):248–250. doi: 10.1038/329248a0. [DOI] [PubMed] [Google Scholar]
- Rance M., Sørensen O. W., Bodenhausen G., Wagner G., Ernst R. R., Wüthrich K. Improved spectral resolution in cosy 1H NMR spectra of proteins via double quantum filtering. Biochem Biophys Res Commun. 1983 Dec 16;117(2):479–485. doi: 10.1016/0006-291x(83)91225-1. [DOI] [PubMed] [Google Scholar]
- Rayfield M., De Cock K., Heyward W., Goldstein L., Krebs J., Kwok S., Lee S., McCormick J., Moreau J. M., Odehouri K. Mixed human immunodeficiency virus (HIV) infection in an individual: demonstration of both HIV type 1 and type 2 proviral sequences by using polymerase chain reaction. J Infect Dis. 1988 Dec;158(6):1170–1176. doi: 10.1093/infdis/158.6.1170. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G. D., Gierasch L. M., Smith J. A. Turns in peptides and proteins. Adv Protein Chem. 1985;37:1–109. doi: 10.1016/s0065-3233(08)60063-7. [DOI] [PubMed] [Google Scholar]
- Schrier R. D., Gnann J. W., Jr, Langlois A. J., Shriver K., Nelson J. A., Oldstone M. B. B- and T-lymphocyte responses to an immunodominant epitope of human immunodeficiency virus. J Virol. 1988 Aug;62(8):2531–2536. doi: 10.1128/jvi.62.8.2531-2536.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. S., Naso R. B., Rosen J., Whalley A., Hom Y. L., Hoey K., Kennedy C. J., McCutchan J. A., Spector S. A., Richman D. D. Antibody to a synthetic oligopeptide in subjects at risk for human immunodeficiency virus infection. J Clin Microbiol. 1987 Aug;25(8):1498–1504. doi: 10.1128/jcm.25.8.1498-1504.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder R. S., O'Connor T., Hughes A., N'jie H., Corrah T., Whittle H. Envelope cross-reactivity in Western blot for HIV-1 and HIV-2 may not indicate dual infection. Lancet. 1988 Oct 22;2(8617):927–930. doi: 10.1016/s0140-6736(88)92598-6. [DOI] [PubMed] [Google Scholar]
- Wagner G., Neuhaus D., Wörgötter E., Vasák M., Kägi J. H., Wüthrich K. Nuclear magnetic resonance identification of "half-turn" and 3(10)-helix secondary structure in rabbit liver metallothionein-2. J Mol Biol. 1986 Jan 5;187(1):131–135. doi: 10.1016/0022-2836(86)90413-4. [DOI] [PubMed] [Google Scholar]
- Wang J. J., Steel S., Wisniewolski R., Wang C. Y. Detection of antibodies to human T-lymphotropic virus type III by using a synthetic peptide of 21 amino acid residues corresponding to a highly antigenic segment of gp41 envelope protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6159–6163. doi: 10.1073/pnas.83.16.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P. E., Dyson H. J., Lerner R. A. Conformation of peptide fragments of proteins in aqueous solution: implications for initiation of protein folding. Biochemistry. 1988 Sep 20;27(19):7167–7175. doi: 10.1021/bi00419a001. [DOI] [PubMed] [Google Scholar]



