Abstract
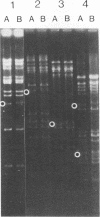
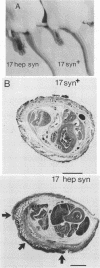
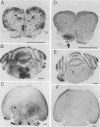
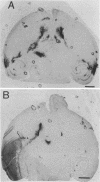
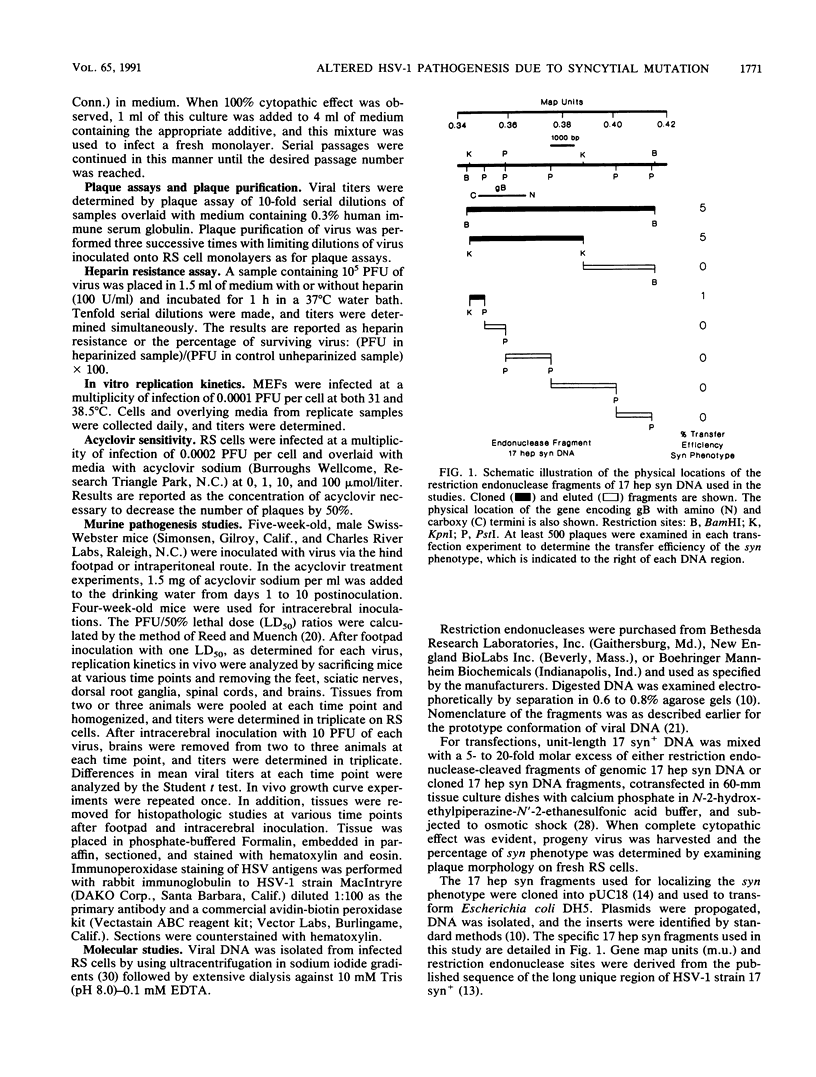
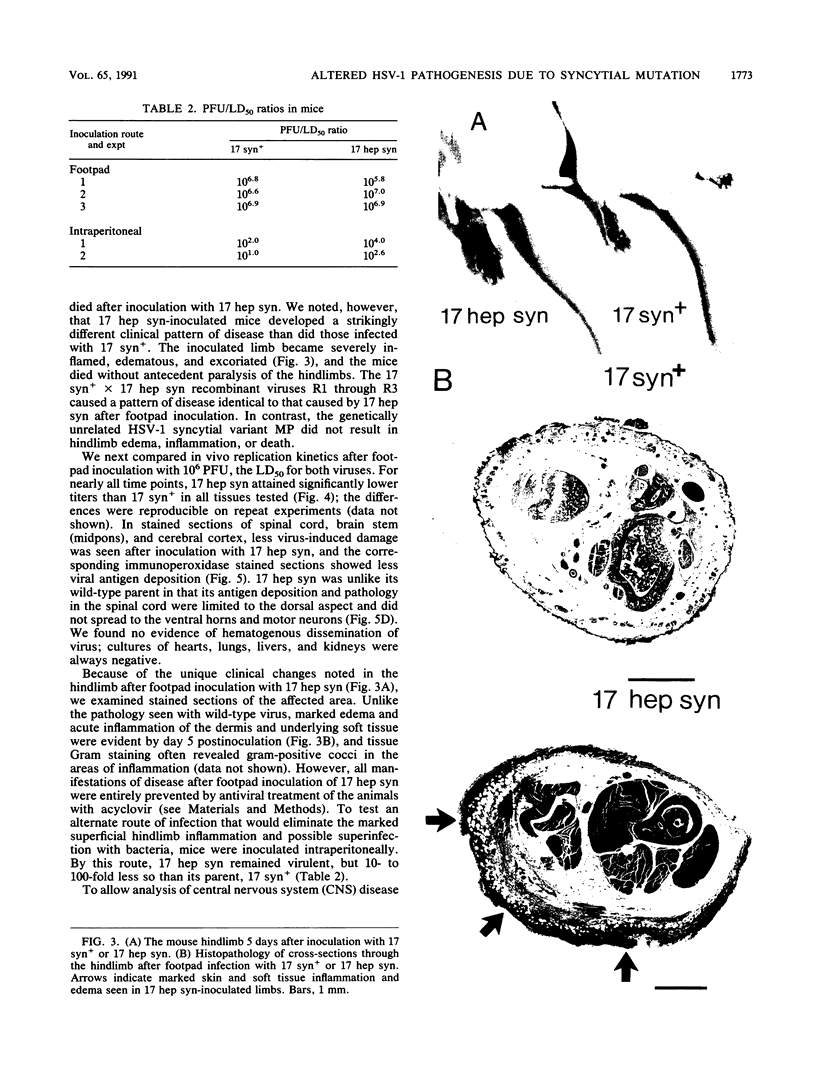
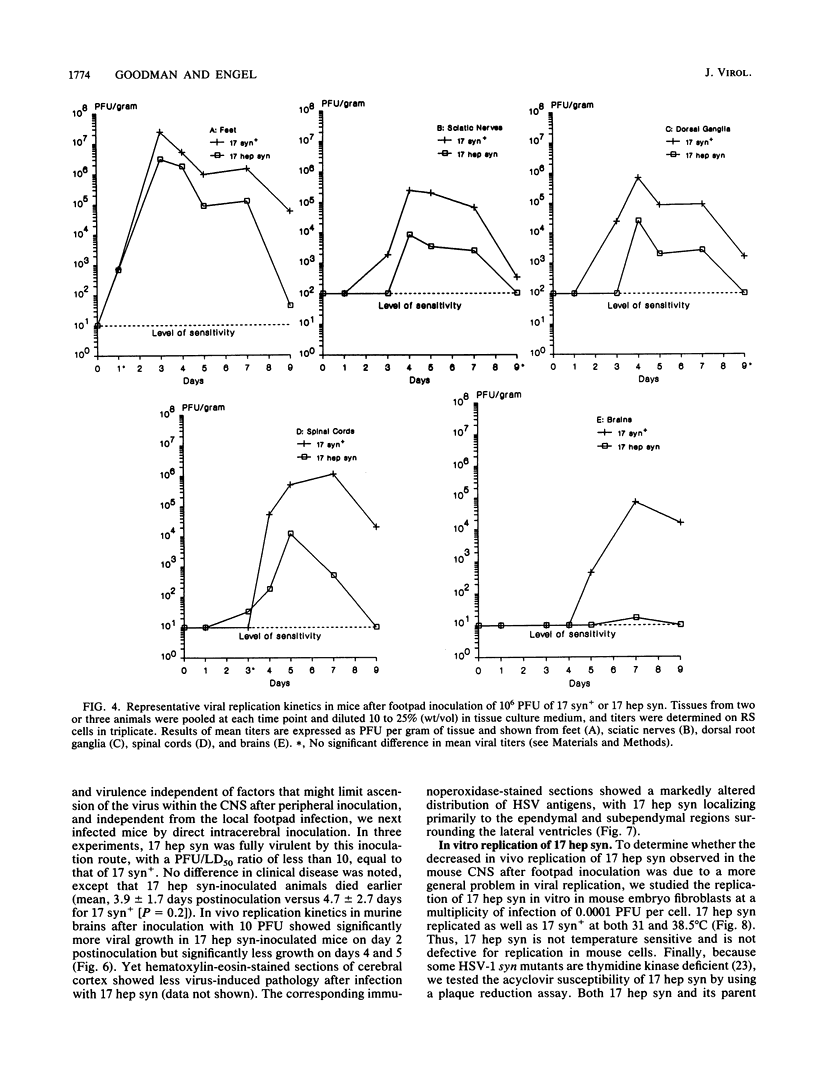
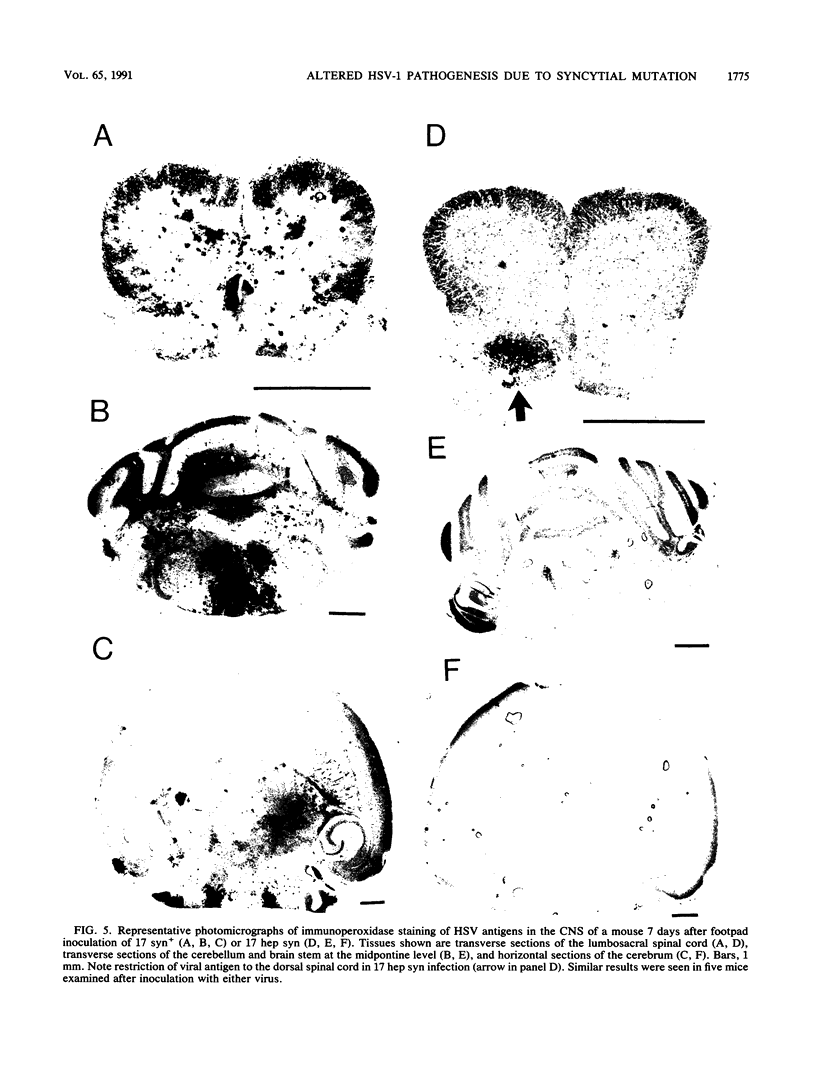
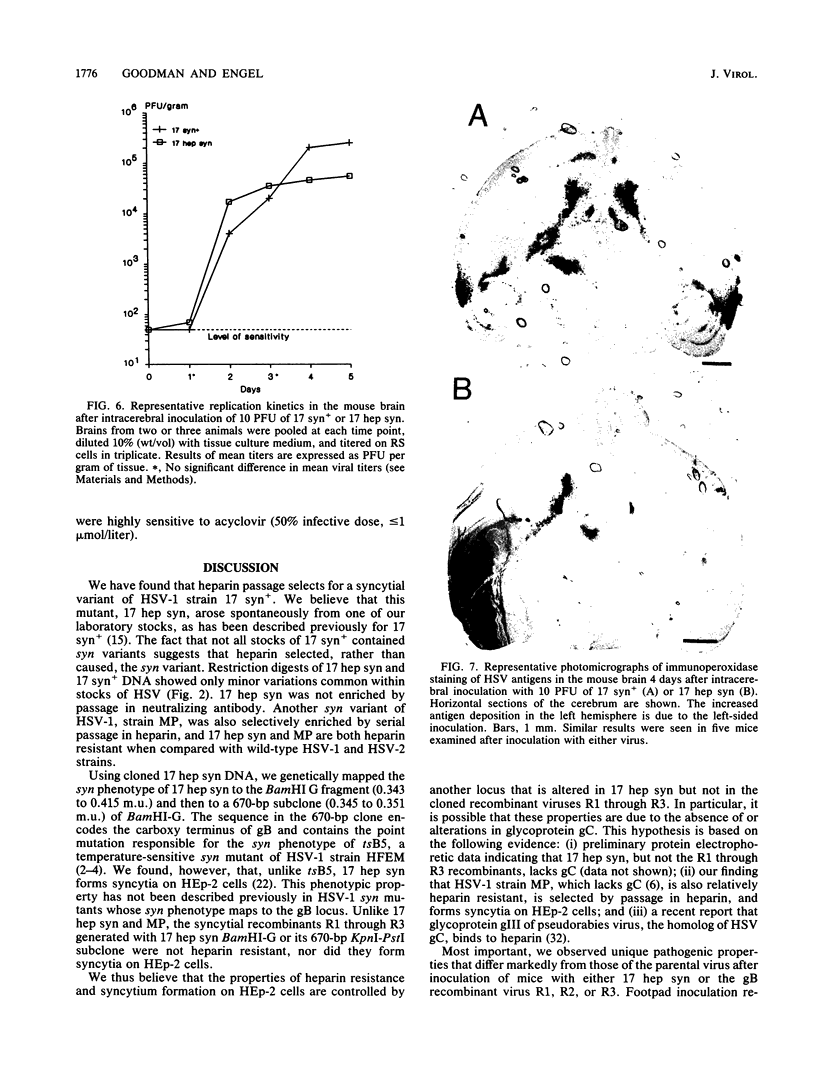
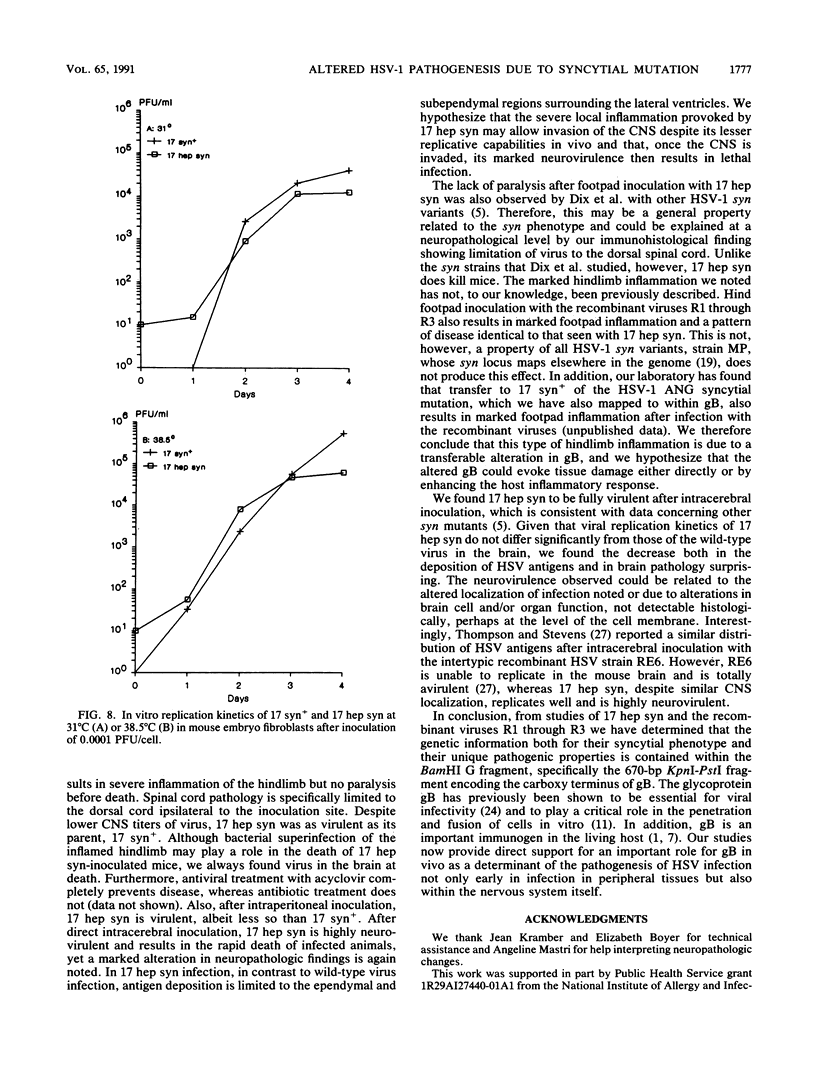
A syncytial (syn) variant of herpes simplex virus type 1 strain 17 syn+ was selected by serial passage in heparin, a glycosaminoglycan which potently inhibits herpes simplex virus infectivity. This virus, 17 hep syn, is sixfold more heparin resistant than its parent. By using marker transfer techniques, its syn phenotype, but not heparin resistance, was mapped first to the BamHI G fragment (0.343 to 0.415 map units) and then to a 670-bp KpnI-PstI subclone (0.345 to 0.351 map units) encoding the carboxy terminus of glycoprotein B (gB). Three cloned syncytial recombinants were generated from cotransfections of 17 syn+ with either 17 hep syn BamHI-G or the 670-bp subclone. After footpad inoculation of mice, 17 hep syn was as virulent as its parent, despite reaching lower titers in feet, sciatic nerves, dorsal root ganglia, spinal cords, and brains. Animals infected with 17 hep syn or the gB recombinant viruses developed a unique pattern of disease that was strikingly different than that seen with wild-type virus: severe inflammation and edema of the inoculated limb and death without antecedent paralysis. Histopathologic examination revealed limitation of spinal involvement by 17 hep syn to the dorsal aspect of the cord and decreased virus-induced damage in the central nervous system. The genetically unrelated syn variant MP, in contrast, was avirulent and did not cause severe local inflammation. After intracerebral inoculation, 17 hep syn was highly virulent and replicated to high titers in the brain. Yet, unlike the parental virus, it resulted in an altered distribution of herpes simplex virus antigens, which were limited to the ependymal and subependymal regions surrounding the lateral ventricles. Despite their syncytial phenotype and pathogenic properties, the recombinant viruses, unlike 17 hep syn, were not heparin resistant. We conclude that a transferable alteration in the 670-bp carboxy-terminal portion of the glycoprotein gB gene of 17 hep syn results in both its syncytial phenotype and the unique pattern of disease that it causes but does not result in heparin resistance. These observations provide direct biological evidence for an important role for herpes simplex virus gB in pathogenic events both at the peripheral site of infection and within the nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blacklaws B. A., Nash A. A., Darby G. Specificity of the immune response of mice to herpes simplex virus glycoproteins B and D constitutively expressed on L cell lines. J Gen Virol. 1987 Apr;68(Pt 4):1103–1114. doi: 10.1099/0022-1317-68-4-1103. [DOI] [PubMed] [Google Scholar]
- Brown S. M., Ritchie D. A., Subak-Sharpe J. H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973 Mar;18(3):329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology. 1984 Aug;137(1):185–190. doi: 10.1016/0042-6822(84)90022-9. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Fox B. A., DeLuca N. A., Person S. Nucleotide sequence specifying the glycoprotein gene, gB, of herpes simplex virus type 1. Virology. 1984 Mar;133(2):301–314. doi: 10.1016/0042-6822(84)90397-0. [DOI] [PubMed] [Google Scholar]
- DeLuca N., Bzik D. J., Bond V. C., Person S., Snipes W. Nucleotide sequences of herpes simplex virus type 1 (HSV-1) affecting virus entry, cell fusion, and production of glycoprotein gb (VP7). Virology. 1982 Oct 30;122(2):411–423. doi: 10.1016/0042-6822(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Dix R. D., McKendall R. R., Baringer J. R. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect Immun. 1983 Apr;40(1):103–112. doi: 10.1128/iai.40.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper K. G., Costa R. H., Lee G. T., Spear P. G., Wagner E. K. Molecular basis of the glycoprotein-C-negative phenotype of herpes simplex virus type 1 macroplaque strain. J Virol. 1984 Sep;51(3):578–585. doi: 10.1128/jvi.51.3.578-585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Hochberg E., Becker Y. Adsorption, penetration and uncoating of herpes simplex virus. J Gen Virol. 1968 Mar;2(2):231–241. doi: 10.1099/0022-1317-2-2-231. [DOI] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks-Hellman S., Ho M. Use of biological characteristics to type Herpesvirus hominis types 1 and 2 in diagnostic laboratories. J Clin Microbiol. 1976 Mar;3(3):277–280. doi: 10.1128/jcm.3.3.277-280.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Dowdle W. R., Kramer J. H., Luce C. F., Mansour S. C. Antibodies to Herpesvirus hominis types 1 and 2 in the rabbit. J Immunol. 1969 Apr;102(4):956–962. [PubMed] [Google Scholar]
- Nahmias A. J., Kibrick S. Inhibitory effect of heparin on herpes simplex virus. J Bacteriol. 1964 May;87(5):1060–1066. doi: 10.1128/jb.87.5.1060-1066.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer G., Waner J. L., Bowling C. P. Comparative studies of type 1 and type 2 & 'herpes simplex' viruses. Br J Exp Pathol. 1968 Apr;49(2):202–208. [PMC free article] [PubMed] [Google Scholar]
- Pogue-Geile K. L., Spear P. G. The single base pair substitution responsible for the Syn phenotype of herpes simplex virus type 1, strain MP. Virology. 1987 Mar;157(1):67–74. doi: 10.1016/0042-6822(87)90314-x. [DOI] [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Ruyechan W. T., Morse L. S., Knipe D. M., Roizman B. Molecular genetics of herpes simplex virus. II. Mapping of the major viral glycoproteins and of the genetic loci specifying the social behavior of infected cells. J Virol. 1979 Feb;29(2):677–697. doi: 10.1128/jvi.29.2.677-697.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P. G., Wilkie N. M., Davison A. J. Thymidine kinase deletion mutants of herpes simplex virus type 1. J Gen Virol. 1982 Dec;63(2):277–295. doi: 10.1099/0022-1317-63-2-277. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subak-Sharpe J. H., Brown S. M., Ritchie D. A., Timbury M. C., Macnab J. C., Marsden H. S., Hay J. Genetic and biochemical studies with herpesvirus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):717–730. doi: 10.1101/sqb.1974.039.01.085. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO K. K., FABISCH P. INHIBITION OF HERPES VIRUS BY NATURAL AND SYNTHETIC ACID POLYSACCHARIDES. Proc Soc Exp Biol Med. 1964 May;116:140–144. doi: 10.3181/00379727-116-29183. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Stevens J. G. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology. 1983 Nov;131(1):171–179. doi: 10.1016/0042-6822(83)90543-3. [DOI] [PubMed] [Google Scholar]
- Thompson R. L., Wagner E. K., Stevens J. G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983 Nov;131(1):180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- VAHERI A., CANTELL K. THE EFFECT OF HEPARIN ON HERPES SIMPLEX VIRUS. Virology. 1963 Dec;21:661–662. doi: 10.1016/0042-6822(63)90242-3. [DOI] [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann F., Zsak L., Reilly L., Sugg N., Ben-Porat T. Early interactions of pseudorabies virus with host cells: functions of glycoprotein gIII. J Virol. 1989 Aug;63(8):3323–3329. doi: 10.1128/jvi.63.8.3323-3329.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]