Abstract
We studied the mechanism of in vitro spontaneous lymphokine production by spleen cells from mice injected intraperitoneally with murine coronavirus stain JHM 1 month after infection, when infectious virus had already been cleared from the spleens. Removal of either CD4+ T cells or Ia+ antigen-presenting cells (APC) from the spleen cells abrogated interleukin-2 (IL-2) production. Addition of anti-CD4 or anti-Iad monoclonal antibodies to the culture suppressed IL-2 production. These results suggest that the response involved typical receptor-mediated activation of T cells. Surprisingly, reciprocal mixing experiments with a coculture of T cells from infected mice and APC from either infected or naive mice resulted in the production of IL-2. The absence of viral antigens in spleen cells 1 month after infection, as indicated by their inability to induce the proliferation of T-cell clones specific for the viral antigens, suggest that the T cells from mice 1 month after infection were not responding to the viral antigens. The inoculum components other than the virus did not induce this immune response. We also found that the frequency of self-reactive but not alloreactive IL-2-producing T cells in the spleens of infected mice was 3- to 10-fold higher than that in naive mice. These findings suggest that an increased frequency of self-reactive T cells which secrete IL-2 occurs following murine coronavirus infection. This may have important implications in the development of autoimmunelike phenomena following murine coronavirus infection.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akimaru K., Stuhlmiller G. M., Seigler H. F. Influence of mouse hepatitis virus on the growth of human melanoma in the peritoneal cavity of the athymic mouse. J Surg Oncol. 1981;17(4):327–339. doi: 10.1002/jso.2930170405. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Dempsey W. L., Smith A. L., Morahan P. S. Effect of inapparent murine hepatitis virus infections on macrophages and host resistance. J Leukoc Biol. 1986 May;39(5):559–565. doi: 10.1002/jlb.39.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon M. T., Schoeb T. R., Benjamin W. H., Jr, Lindsey J. R., Briles D. E. Modulation of resistance to Salmonella typhimurium infection in mice by mouse hepatitis virus (MHV). Microb Pathog. 1989 Feb;6(2):81–91. doi: 10.1016/0882-4010(89)90011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. O., Trousdale M. D., Bradbury J., Stohlman S. A., Weiner L. P. Experimental demyelination induced by coronavirus JHM (MHV-4): molecular identification of a viral determinant of paralytic disease. Microb Pathog. 1987 Jul;3(1):9–20. doi: 10.1016/0882-4010(87)90033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E. K., Lo D., Cheney R., Kanagawa O., Sprent J. Abnormal differentiation of thymocytes in mice treated with cyclosporin A. Nature. 1988 Nov 10;336(6195):176–179. doi: 10.1038/336176a0. [DOI] [PubMed] [Google Scholar]
- Glazier A., Tutschka P. J., Farmer E. R., Santos G. W. Graft-versus-host disease in cyclosporin A-treated rats after syngeneic and autologous bone marrow reconstitution. J Exp Med. 1983 Jul 1;158(1):1–8. doi: 10.1084/jem.158.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. D., Horwitz L., Beschorner W. E., Santos G. W. Development of graft-vs.-host disease-like syndrome in cyclosporine-treated rats after syngeneic bone marrow transplantation. I. Development of cytotoxic T lymphocytes with apparent polyclonal anti-Ia specificity, including autoreactivity. J Exp Med. 1985 Apr 1;161(4):718–730. doi: 10.1084/jem.161.4.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton A., Mizzen L., MacIntyre G., Cheley S., Anderson R. Translational control in murine hepatitis virus infection. J Gen Virol. 1986 May;67(Pt 5):923–932. doi: 10.1099/0022-1317-67-5-923. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H., Pardoll D. M. Effects of cyclosporine A on T cell development and clonal deletion. Science. 1988 Sep 23;241(4873):1655–1658. doi: 10.1126/science.241.4873.1655. [DOI] [PubMed] [Google Scholar]
- Jennings S. R., Rice P. L., Kloszewski E. D., Anderson R. W., Thompson D. L., Tevethia S. S. Effect of herpes simplex virus types 1 and 2 on surface expression of class I major histocompatibility complex antigens on infected cells. J Virol. 1985 Dec;56(3):757–766. doi: 10.1128/jvi.56.3.757-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kasaian M. T., Biron C. A. The activation of IL-2 transcription in L3T4+ and Lyt-2+ lymphocytes during virus infection in vivo. J Immunol. 1989 Feb 15;142(4):1287–1292. [PubMed] [Google Scholar]
- Keck J. G., Matsushima G. K., Makino S., Fleming J. O., Vannier D. M., Stohlman S. A., Lai M. M. In vivo RNA-RNA recombination of coronavirus in mouse brain. J Virol. 1988 May;62(5):1810–1813. doi: 10.1128/jvi.62.5.1810-1813.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatchikian D., Orlich M., Rott R. Increased viral pathogenicity after insertion of a 28S ribosomal RNA sequence into the haemagglutinin gene of an influenza virus. Nature. 1989 Jul 13;340(6229):156–157. doi: 10.1038/340156a0. [DOI] [PubMed] [Google Scholar]
- Knobler R. L., Oldstone M. B. Infection and involution of mouse thymus by MHV-4. Adv Exp Med Biol. 1987;218:451–453. doi: 10.1007/978-1-4684-1280-2_56. [DOI] [PubMed] [Google Scholar]
- Kyriazis A. P., DiPersio L., Michael J. G., Pesce A. J. Influence of the mouse hepatitis virus (MHV) infection on the growth of human tumors in the athymic mouse. Int J Cancer. 1979 Mar 15;23(3):402–409. doi: 10.1002/ijc.2910230320. [DOI] [PubMed] [Google Scholar]
- Kyuwa S., Yamaguchi K., Fujiwara K., Yamanouchi K. Bone marrow cell-dependency of delayed-type hypersensitivity response in mice infected with mouse hepatitis virus. Jpn J Exp Med. 1986 Jun;56(3):93–98. [PubMed] [Google Scholar]
- Kyuwa S., Yamaguchi K., Hayami M., Fujiwara K. Characterization of mouse hepatitis virus-reactive T cell clones. Adv Exp Med Biol. 1987;218:391–398. doi: 10.1007/978-1-4684-1280-2_48. [DOI] [PubMed] [Google Scholar]
- Kyuwa S., Yamaguchi K., Hayami M., Hilgers J., Fujiwara K. Spontaneous production of interleukin-2 and interleukin-3 by spleen cells from mice infected with mouse hepatitis virus type 4. J Virol. 1988 Sep;62(9):3506–3508. doi: 10.1128/jvi.62.9.3506-3508.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattime E. C., Stutman O. L3T4+, B2A2+ thymocytes from infant mice produce IL 2 after interaction with accessory cells expressing self class II antigens. J Immunol. 1986 Apr 15;136(8):2741–2746. [PubMed] [Google Scholar]
- Ly I. A., Mishell R. I. Separation of mouse spleen cells by passage through columns of sephadex G-10. J Immunol Methods. 1974 Aug;5(3):239–247. doi: 10.1016/0022-1759(74)90108-2. [DOI] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Makino S., Keck J. G., Stohlman S. A., Lai M. M. High-frequency RNA recombination of murine coronaviruses. J Virol. 1986 Mar;57(3):729–737. doi: 10.1128/jvi.57.3.729-737.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa P. T., Dörries R., ter Meulen V. Viral particles induce Ia antigen expression on astrocytes. Nature. 1986 Apr 10;320(6062):543–546. doi: 10.1038/320543a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McChesney M. B., Oldstone M. B. Virus-induced immunosuppression: infections with measles virus and human immunodeficiency virus. Adv Immunol. 1989;45:335–380. doi: 10.1016/s0065-2776(08)60696-3. [DOI] [PubMed] [Google Scholar]
- McChesney M. B., Oldstone M. B. Viruses perturb lymphocyte functions: selected principles characterizing virus-induced immunosuppression. Annu Rev Immunol. 1987;5:279–304. doi: 10.1146/annurev.iy.05.040187.001431. [DOI] [PubMed] [Google Scholar]
- Midoro K., Nakanaga K., Kyuwa S., Fujiwara K. Immunopathology of chronic progressive hepatitis in nude mice infected with low-virulent mouse hepatitis virus. Microbiol Immunol. 1989;33(8):669–682. doi: 10.1111/j.1348-0421.1989.tb02017.x. [DOI] [PubMed] [Google Scholar]
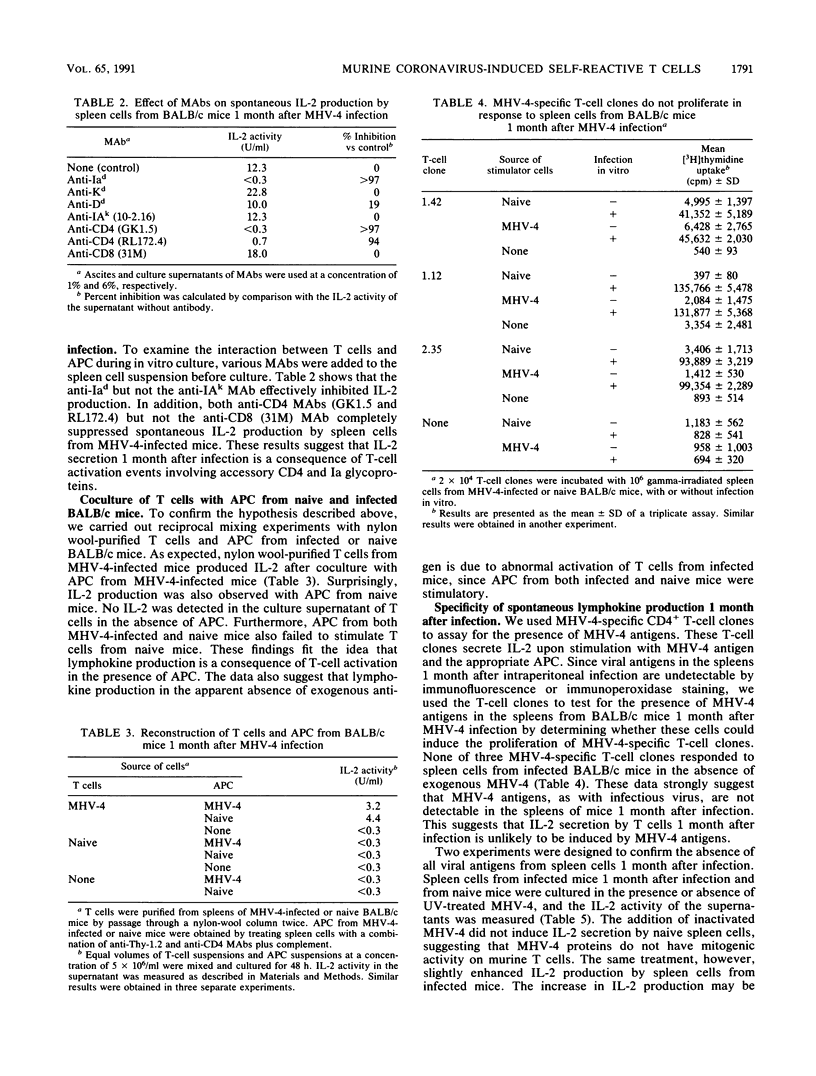
- Miller R. A., Stutman O. Enumeration of IL 2-secreting helper T cells by limiting dilution analysis, and demonstration of unexpectedly high levels of IL 2 production per responding cell. J Immunol. 1982 May;128(5):2258–2264. [PubMed] [Google Scholar]
- Mims C. A. Interactions of viruses with the immune system. Clin Exp Immunol. 1986 Oct;66(1):1–16. [PMC free article] [PubMed] [Google Scholar]
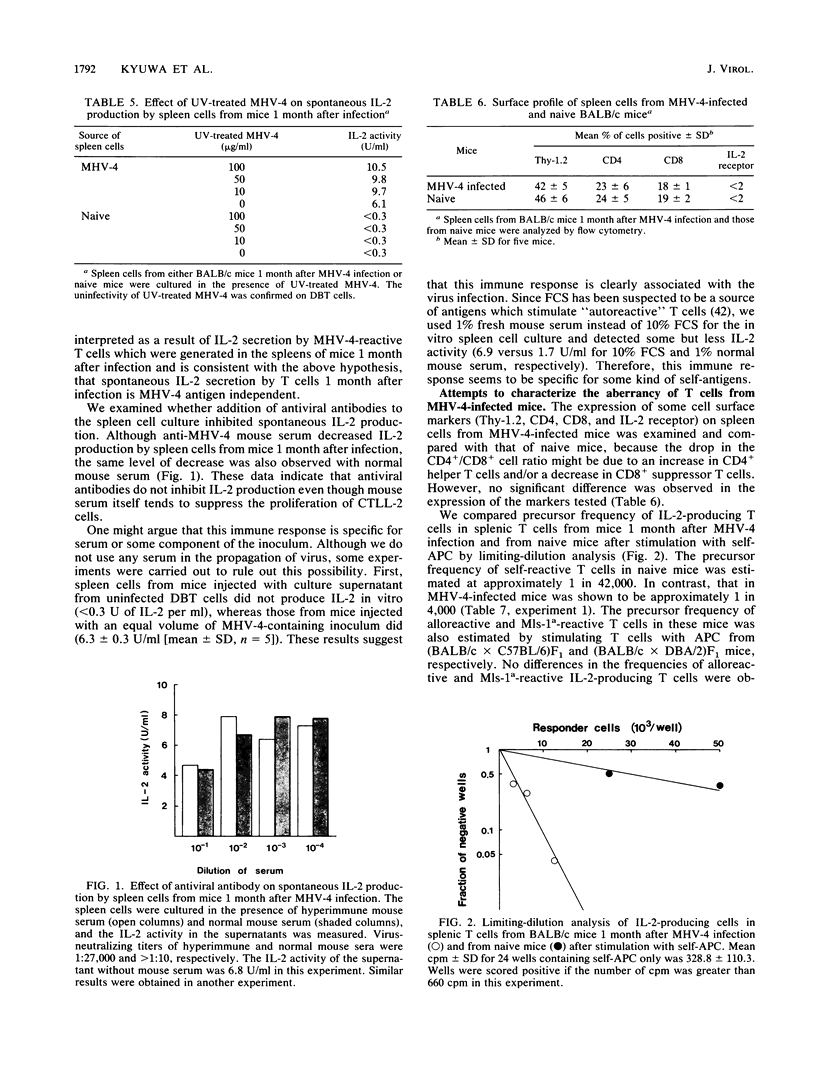
- Monroe S. S., Schlesinger S. RNAs from two independently isolated defective interfering particles of Sindbis virus contain a cellular tRNA sequence at their 5' ends. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3279–3283. doi: 10.1073/pnas.80.11.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
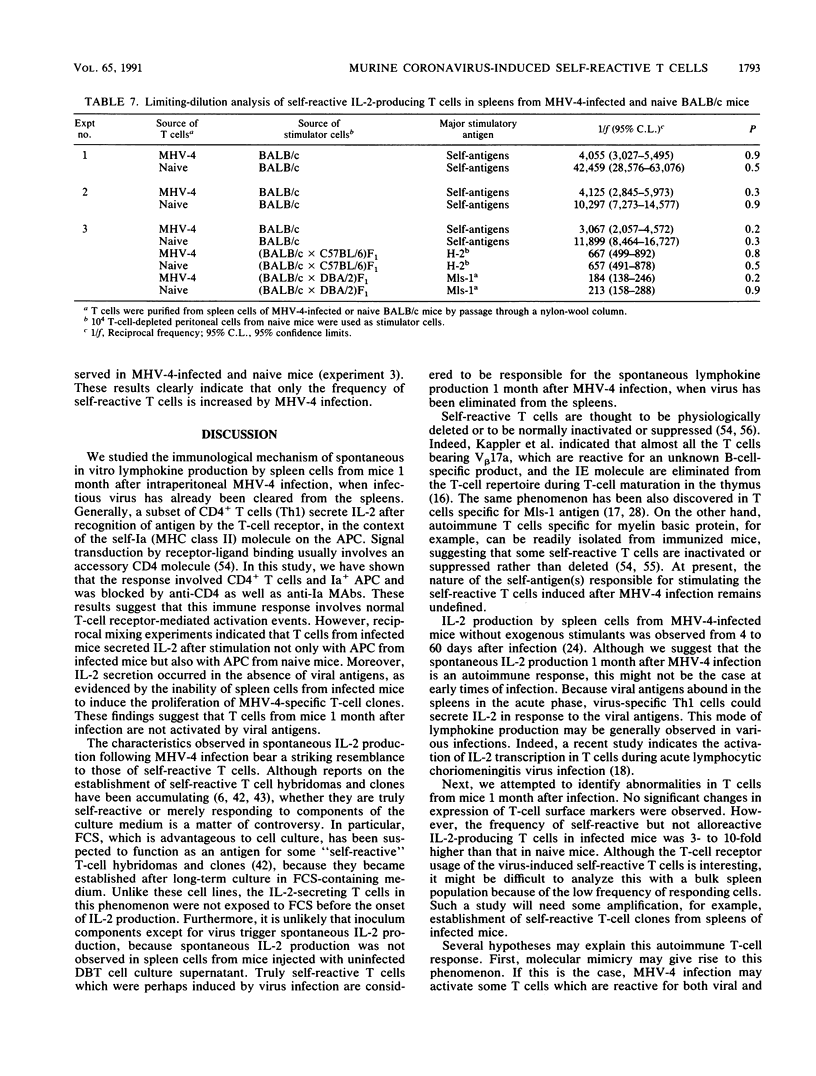
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Oleszak E. L., Leibowitz J. L. Immunoglobulin Fc binding activity is associated with the mouse hepatitis virus E2 peplomer protein. Virology. 1990 May;176(1):70–80. doi: 10.1016/0042-6822(90)90231-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER E. H., BERRY R. J. THE EFFICIENT DESIGN OF TRANSPLANTABLE TUMOUR ASSAYS. Br J Cancer. 1963 Dec;17:583–595. doi: 10.1038/bjc.1963.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. E., Gallagher T. M., Buchmeier M. J. Sequence analysis reveals extensive polymorphism and evidence of deletions within the E2 glycoprotein gene of several strains of murine hepatitis virus. Virology. 1989 Dec;173(2):664–673. doi: 10.1016/0042-6822(89)90579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen A. M., Munro A. J. Fetal calf serum stimulates 'autoreactive' T-cell hybridomas. Immunology. 1988 Feb;63(2):255–260. [PMC free article] [PubMed] [Google Scholar]
- Reimann J., Bellan A., Conradt P. Development of autoreactive L3T4+ T cells from double-negative (L3T4-/Ly-2-) Thy-1+ spleen cells of normal mice. Eur J Immunol. 1988 Jul;18(7):989–999. doi: 10.1002/eji.1830180704. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Glasebrook A. L., Fitch F. W. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980 Dec;125(6):2665–2672. [PubMed] [Google Scholar]
- Schedi M. P., Goldstein G., Boyce E. A. Differentiation of T cells in nude mice. Science. 1975 Dec 19;190(4220):1211–1213. [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorokin R., Kimura H., Schroder K., Wilson D. H., Wilson D. B. Cyclosporine-induced autoimmunity. Conditions for expressing disease, requirement for intact thymus, and potency estimates of autoimmune lymphocytes in drug-treated rats. J Exp Med. 1986 Nov 1;164(5):1615–1625. doi: 10.1084/jem.164.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura A., Lavi E., Weiss S. R., Silberberg D. H. Coronavirus infection induces H-2 antigen expression on oligodendrocytes and astrocytes. Science. 1986 May 23;232(4753):991–993. doi: 10.1126/science.3010460. [DOI] [PubMed] [Google Scholar]
- Taguchi F., Fleming J. O. Comparison of six different murine coronavirus JHM variants by monoclonal antibodies against the E2 glycoprotein. Virology. 1989 Mar;169(1):233–235. doi: 10.1016/0042-6822(89)90061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Fujiwara K. IgM and IgG response to sheep red blood cells in mouse hepatitis virus-infected nude mice. Microbiol Immunol. 1979;23(3):177–183. doi: 10.1111/j.1348-0421.1979.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Tamura T., Machii K., Ueda K., Fujiwara K. Modification of immune response in nude mice infected with mouse hepatitis virus. Microbiol Immunol. 1978;22(9):557–564. doi: 10.1111/j.1348-0421.1978.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Watanabe R., Wege H., ter Meulen V. Adoptive transfer of EAE-like lesions from rats with coronavirus-induced demyelinating encephalomyelitis. Nature. 1983 Sep 8;305(5930):150–153. doi: 10.1038/305150a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Siddell S., ter Meulen V. The biology and pathogenesis of coronaviruses. Curr Top Microbiol Immunol. 1982;99:165–200. doi: 10.1007/978-3-642-68528-6_5. [DOI] [PubMed] [Google Scholar]
- Wraith D. C., McDevitt H. O., Steinman L., Acha-Orbea H. T cell recognition as the target for immune intervention in autoimmune disease. Cell. 1989 Jun 2;57(5):709–715. doi: 10.1016/0092-8674(89)90786-1. [DOI] [PubMed] [Google Scholar]
- Zamvil S., Nelson P., Trotter J., Mitchell D., Knobler R., Fritz R., Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. 1985 Sep 26-Oct 2Nature. 317(6035):355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- Zauderer M. Origin and significance of autoreactive T cells. Adv Immunol. 1989;45:417–437. doi: 10.1016/s0065-2776(08)60698-7. [DOI] [PubMed] [Google Scholar]
- de Talance A., Regnier D., Spinella S., Morisset J., Seman M. Origin of autoreactive T helper cells. I. Characterization of Thy-1+, Lyt-, L3T4- precursors in the spleen of normal mice. J Immunol. 1986 Aug 15;137(4):1101–1108. [PubMed] [Google Scholar]



