Abstract
A gene, sirZ, encoding a Zn(II)2Cys6 DNA binding protein is present in a cluster of genes responsible for the biosynthesis of the epipolythiodioxopiperazine (ETP) toxin, sirodesmin PL in the ascomycete plant pathogen, Leptosphaeria maculans. RNA-mediated silencing of sirZ gives rise to transformants that produce only residual amounts of sirodesmin PL and display a decrease in the transcription of several sirodesmin PL biosynthetic genes. This indicates that SirZ is a major regulator of this gene cluster. Proteins similar to SirZ are encoded in the gliotoxin biosynthetic gene cluster of Aspergillus fumigatus (gliZ) and in an ETP-like cluster in Penicillium lilacinoechinulatum (PlgliZ). Despite its high level of sequence similarity to gliZ, PlgliZ is unable to complement the gliotoxin-deficiency of a mutant of gliZ in A. fumigatus. Putative binding sites for these regulatory proteins in the promoters of genes in these clusters were predicted using bioinformatic analysis. These sites are similar to those commonly bound by other proteins with Zn(II)2Cys6 DNA binding domains.
Keywords: Toxin, Aspergillus, Leptosphaeria, gliotoxin, sirodesmin, ETP, Zn(II)2Cys6
1. Introduction
Sirodesmin PL is the major phytotoxin produced by the plant pathogen Leptosphaeria maculans (Desm.), the causal agent of blackleg disease of Brassica napus (canola). Sirodesmin PL has antibacterial and antiviral properties (Rouxel et al., 1988) and is essential for full virulence of L. maculans on stems of Brassica napus (Elliott et al., 2007). This toxin is a member of the epipolythiodioxopiperazine (ETP) class of secondary metabolites. Epipolythiodioxopiperazines are characterised by the presence of a disulphide-bridged dioxopiperazine ring synthesised from two amino acids (for review see Gardiner et al., 2005). The best-characterised ETP is gliotoxin, which is produced by the opportunistic human pathogen Aspergillus fumigatus, as well as by other ascomycetes A. terreus, A. flavus, A. niger (Lewis et al., 2005), Penicillium terkilowskii (Waring et al., 1987), Trichoderma virens (syn. Gliocladium virens) (Wilhite et al., 1994) and Trichoderma viride (Bu'Lock and Leigh, 1975).
The genes that encode enzymes for biosynthesis of fungal secondary metabolites are usually clustered (Keller and Hohn, 1997). A cluster of 18 genes responsible for the biosynthesis of sirodesmin PL in L. maculans has been identified (Gardiner et al., 2004) (Fig. 1). Disruption of the peptide synthetase encoded in the cluster results in an isolate unable to produce sirodesmin PL, confirming the role of this protein in the biosynthesis of this toxin. Transcription of nine genes in the cluster were tested and all were co-regulated with the production of sirodesmin PL in culture, suggesting the gene cluster is responsible for the biosynthesis of sirodesmin PL (Gardiner et al., 2004). Based on comparative genomics, the cluster of genes responsible for the biosynthesis of gliotoxin in A. fumigatus was then predicted (Gardiner and Howlett, 2005). The identity of this cluster was confirmed via disruption of the peptide synthetase, gliP, whereby the resultant mutant was unable to make gliotoxin (Cramer et al., 2006; Kupfahl et al., 2006). Recently, a putative gliotoxin biosynthetic gene cluster was identified in a strain of a gliotoxin-producing fungus Penicillium lilacinoechinulatum (Patron et al., 2007). However, as functional analysis of this cluster has not been performed, whether this cluster is responsible for gliotoxin biosynthesis is not known. The L. maculans gene cluster has nine and ten genes in common with the P. lilacinoechinulatum and A. fumigatus gene clusters, respectively (Fig. 1). Other genes in the sirodesmin and gliotoxin clusters include those thought to be responsible for modifications of the core moiety (Gardiner and Howlett, 2005).
Fig. 1.
Gene clusters in Leptosphaeria maculans (for biosynthesis of sirodesmin PL), in Aspergillus fumigatus (for biosynthesis of gliotoxin) and in Penicillium lilacinoechinulatum (predicted to be the biosynthetic gene cluster for gliotoxin). Common ETP genes (white text on black background) include those with best matches to non-ribosomal peptide synthetase (P), thioredoxin reductase (T), methyl transferases (M and N), glutathione S-transferase (G), cytochrome P450 mono-oxygenase (C), amino cyclopropane carboxylate synthase (ACCS) (I), dipeptidase (J), as well as a transcriptional regulator (Z) and a transporter (A). The P. lilacinoechinulatum putative gliotoxin cluster lacks N, but has the nine other genes. Other genes (black text on white background) do not have obvious homologues in all three gene clusters and are thought to be involved in modification of the side chains of the core ETP moiety. These encode cytochrome P450 mono-oxygenases (F, B and E), a prenyl transferase (D), an acetyl transferase (H), epimerases (Q, S and R), an oxidoreductase (O) and a hypothetical protein (K) (Gardiner and Howlett, 2005). Genes shaded in grey encode proteins with best matches to proteins with no potential roles in ETP biosynthesis and are predicted to flank the cluster. The names of these genes in the L. maculans gene cluster are written above the corresponding genes. The forward slash marks represent a 17 kb region of repetitive DNA in A. fumigatus.
In addition to being physically clustered in the genome, genes responsible for the biosynthesis of secondary metabolites are often co-ordinately regulated in filamentous fungi. This co-regulation can, in part, be explained by coordinated transcriptional control of biosynthetic genes by pathway-specific regulators. Such regulators are specific to a particular metabolic pathway and are often encoded within the gene cluster. Many pathway-specific regulatory proteins contain a Zn(II)2Cys6 DNA binding domain, which is unique to fungi. The archetypal protein with this domain is AflR, which is required for activation of aflatoxin and sterigmatocystin biosynthetic genes in Aspergillus spp. (Chang et al., 1995; Fernandes et al., 1998; Woloshuk et al., 1994; Yu et al., 1996). The fumonisin biosynthetic gene cluster in Fusarium verticillioides also encodes a Zn(II)2Cys6 protein that affects transcription of the cluster genes and fumonisin production (Brown et al., 2007). Another Zn(II)2Cys6 transcriptional activator, CTB8, regulates the transcription of seven clustered genes responsible for the biosynthesis of cercosporin in Cercospora nicotianae (Chen et al., 2007). The aurofusarin biosynthetic gene cluster in Gibberella zeae is regulated by the Zn(II)2Cys6 protein, GIP2 (Kim et al., 2006). Zn(II)2Cys6 DNA binding domains interact with DNA binding sites commonly consisting of conserved terminal trinucleotides, which are usually in a symmetrical configuration and are spaced by an internal variable sequence of defined length, for example, CCG(N)XCGG (Todd and Andrianopoulos, 1997).
The afore-mentioned gene clusters in L. maculans, A. fumigatus and P. lilacinoechinulatum each encode a protein with homology to the Zn(II)2Cys6 class of transcriptional regulators. These are SirZ, GliZ and PlGliZ, respectively. The presence of this binding domain in all three predicted protein sequences suggests a role in transcriptional regulation. Indeed, disruption of gliZ in A. fumigatus resulted in a mutant, ΔgliZ, that had significantly reduced transcription of the gliotoxin biosynthetic gene, gliI and was unable to produce gliotoxin (Bok et al., 2006).
In this paper we describe the characterisation of sirZ from Leptosphaeria maculans. Due to the extreme difficulty of gene disruption in L. maculans, RNA-mediated silencing, which is commonly used to silence gene expression in fungi (eg. Cardoza et al., 2007; Fitzgerald et al., 2004; McDonald et al., 2005; Schumann and Hertweck, 2007), was employed to investigate the effect of loss of sirZ on the production of sirodesmin PL. Silenced mutants were also analysed for the transcription of sirodesmin PL biosynthetic genes, and genes flanking the gene cluster. Given the sequence similarity between L. maculans sirZ, A. fumigatus gliZ and P. lilacinoechinulatum PlgliZ, it was of interest whether the functions of these genes are conserved. To determine this, we attempted to cross-complement the gliotoxin-deficient A. fumigatus ΔgliZ mutant (Bok et al., 2006) with L. maculans sirZ and P. lilacinoechinulatum PlgliZ. The resulting transformants were tested for gliotoxin production and the transcription of gliotoxin biosynthetic genes. We also undertook a computational approach to identify conserved, over-represented DNA motifs in the intergenic regions of the genes in the three clusters that could serve as putative cis-elements.
2. Materials and Methods
2.1. Fungal culturing
All fungal strains used are listed in Table 1. Leptosphaeria maculans isolates were maintained on 10% V8 juice (Campbells, Australia) agar and grown at 22°C with a 12 h/12 h light/dark cycle. Conidia were harvested by flooding plates with sterile water. For liquid cultures, conidia (106) were inoculated into 10% V8 juice (50 mL) and grown at 22°C in the dark, without agitation. Aspergillus fumigatus isolates were maintained on 10% V8 juice agar and grown at 37°C in the dark. Conidia were harvested by flooding plates with sterile 0.5% (v/v) Tween 20. Conidia (107) were inoculated into 10% V8 juice (15 mL), Czapek Dox medium (15 mL), Czapek Dox medium with 0.5% Yeast Extract (15 mL) or glucose minimal medium (GMM) (Shimizu and Keller, 2001) (15 mL) and grown at 37°C or 25°C, with agitation. RNA or genomic DNA was extracted from mycelia, and culture filtrates were analysed for the presence of sirodesmin PL or gliotoxin.
Table 1. Strain list.
| Strains | Species | Genotype |
|---|---|---|
| IBCN 18 | Leptosphaeria maculans | Wild-type |
| sirZRNAi2 | Leptosphaeria maculans | LmsirZ silenced: hygB |
| sirZRNAi4 | Leptosphaeria maculans | LmsirZ silenced: hygB |
| sirZRNAi6 | Leptosphaeria maculans | LmsirZ silenced: hygB |
| ΔsirP | Leptosphaeria maculans | ΔsirP: hyg B |
| Af293 | Aspergillus fumigatus | Wild-type |
| ΔgliZ | Aspergillus fumigatus | ΔgliZ::pyrG pyrG1 (Bok et al., 2006) |
| ΔgliZ:AfgliZ | Aspergillus fumigatus | gliZ hygB ΔgliZ::pyrG pyrG1 (Bok et al., 2006) |
| ΔgliZ:LmsirZ1 | Aspergillus fumigatus | LmsirZ hygB ΔgliZ::pyrG pyrG1 |
| ΔgliZ:LmsirZ2 | Aspergillus fumigatus | LmsirZ hygB ΔgliZ::pyrG pyrG1 |
| ΔgliZ:LmsirZ3 | Aspergillus fumigatus | LmsirZ hygB ΔgliZ::pyrG pyrG1 |
| ΔgliZ:PlgliZ1 | Aspergillus fumigatus | PlgliZ hygB ΔgliZ::pyrG pyrG1 |
| ΔgliZ:PlgliZ2 | Aspergillus fumigatus | PlgliZ hygB ΔgliZ::pyrG pyrG1 |
| ΔgliZ:PlgliZ3 | Aspergillus fumigatus | PlgliZ hygB ΔgliZ::pyrG pyrG1 |
2.2 DNA sequencing and protein alignments
The predicted sequence of the Leptosphaeria maculans sirZ gene was obtained from a previous study (Gardiner et al., 2004) (GenBank Accession no. AY553235). Intron positions and transcriptional start and stop sites were determined using Reverse Transcription-PCR (RT-PCR) and Gene Racer 5′- and 3′ Rapid Amplification of cDNA Ends kit (Invitrogen, USA). Products were cloned (pCR2.1 TOPO-TA cloning kit, Invitrogen) and sequenced. The sequence of the Penicillium lilacinoechinulatum PlgliZ gene was obtained from a previous study (Patron et al., 2007) (GenBank accession no. EF429247). The sequence of Aspergillus fumigatus gliZ was obtained from a previous study (Gardiner and Howlett, 2005) (GenBank accession no. AY838877). Protein sequences were aligned using the CLUSTALW algorithm (Thompson et al., 1994).
2.3 Plasmid construction
Plasmids were constructed using standard molecular techniques. Accuprime High Fidelity Taq Polymerase (Invitrogen) was used for PCR reactions. Primers for PCR and probes are listed in Table 2. To facilitate RNA-mediated silencing of sirZ, a vector was created that allows the generation of hairpin constructs by Gateway® cloning (Invitrogen). This vector, pHYGGS, was constructed in a pBluescript II (Stratagene) base vector. The 192 bp intron of the L. maculans metallothionein-like gene CAP5 (GenBank accession no.AY541064) was PCR-amplified (primers: 5′ CCCAAGCTTCTCGAGTCAAACATCACCATGTCTCC 3′ and 5′ CGCGGATCCTCTAGAACACTTGCAAGAGGCGCAG 3′) from L. maculans genomic DNA and directionally cloned using the HindIII and BamHI sites (italicised) into the vector pBSTrpC, which contains A. nidulans TrpC regulatory sequences (Gardiner et al., 2004). The CAP5 sequence acts as an “intron” in the hairpin structure that is ultimately expressed by this vector. Gateway® sites were introduced into the XhoI and XbaI sites (underlined in primer sequences) sequentially from pHellsgate8 (Helliwell and Waterhouse, 2003) and orientation was checked by restriction digestion. These Gateway® attR1-2 sites facilitate the cloning of DNA fragments in opposing orientation from specific attL-flanked entry clones. All manipulations using Gateway® fragments were carried out in E. coli DB3.1, which is able to maintain the otherwise toxic ccdB gene. The entire gene silencing fragment (promoter and terminator, intron, and inverted repeat of the Gateway® fragments) was restricted with KpnI and SacI subcloned into the EcoRV site of pPZPHyg3, a plasmid containing a hygromycin phosphotransferase selectable marker (Elliott and Howlett, 2006). The resulting vector, pHYGGS, was used as the basis for the sirZ RNA-mediated silencing construct. A region of the sirZ coding sequence was amplified from L. maculans IBCN 18 genomic DNA using attB1 and attB2 tailed primers, sirZRNAiF and sirZRNAiR, respectively. This fragment was cloned into Gateway® vector pDONR207 using BP clonase (Invitrogen), to create pDONRsirZ. The fragment was then moved from pDONRsirZ into pHYGGS in two opposing orientations using LR Clonase (Invitrogen) to create the final sirZ gene silencing vector, psirZRNAi. The vector was transformed into Agrobacterium tumefaciens strain LBA4404 (Invitrogen) according to manufacturers' instructions.
Table 2. Primer list.
| Primer name | Sequence (5′ → 3′) |
|---|---|
| sirZRNAiF | CCGAGAGCGTGTGAACAGTA |
| sirZRNAiR | GAATGCAATCTGCTCTGCAA |
| sirZF | ACGATACGATTCGGCTCAAC |
| SirZR | TGAAATTTGCAGAGCGACAC |
| PlgliZF | ATCAATTTCGCACGGCTTAC |
| PlgliZR | TGAAATTTGCAGAGCGACAC |
| ZnFF | GTCGAATCGGTCCACAACTT |
| ZnFR | TGTGCGCAAATTCATTTCAT |
| HDX1F | CAGCGTGCTACTCAAATCCA |
| HDX1R | AACTCCAGGCTTAGCGATCA |
| sirZ2F | CCGAATTTCCCTTCAGTCAA |
| sirZ1R | CAATGGGTCTGGAATACGCT |
| PlgliZ3F | GTGTCGCTCTGCAAATTTCA |
| PlgliZ3R | GTGTCGCTCTGCAAATTTCA |
| AfpsF | AAACCCCTGTGAATGCAGAC |
| AfpsR | CCCCTTGAGATGAAAGGTGA |
| AfaccsF | AGGCCATCCTCGTGTGTAAC |
| AfaccsR | GCCGAGGTCTTTGCTGATAC |
Vectors for the heterologous complementation of A. fumigatus ΔgliZ isolate were created as follows. A PCR product (3.8 kb) containing sirZ was amplified from genomic DNA of L. maculans isolate IBCN 18 (primers sirZF and sirZR). The amplified fragment included approximately 1 kb of promoter and 0.5 kb of 3′ untranslated region (UTR) sequence. The product was A-tailed (Taq DNA polymerase, Invitrogen), cloned into pGem-T-Easy (Promega) and sequenced to check for errors. The fragment was subcloned into the XbaI and XmaI sites of pUCH2-8, which contains the hygromycin B phosphotransferase gene (Alexander et al., 1998) to create plasmid pLmsirZ. The P. lilacinoechinulatum gliZ gene was cloned as follows. A 4 kb fragment containing PlgliZ was amplified from genomic DNA of P. lilacinoechinulatum lab strain IBT 28164 (primers PlgliZF and PlgliZR). The amplified fragment included approximately 1 kb of promoter and 0.5 kb of 3′UTR sequence. The product was A-tailed, cloned into pGem-T-Easy and sequenced to check for errors. The fragment was subcloned into pUCH2-8, using NotI and ScaI, to create plasmid pPlgliZ.
2.4 Fungal transformation
Conidia of L. maculans wild-type isolate IBCN 18 were transformed using Agrobacterium-mediated DNA delivery (Gardiner and Howlett, 2004). Transformants were subjected to two rounds of selection on hygromycin (50 μg mL-1). Protoplasts of A. fumigatus ΔgliZ mutant were transformed by the polyethylene glycol method as previously described (Bok et al., 2005). Transformants were selected on hygromycin (110 μg mL-1). Southern analysis was used to assess vector copy number in hygromycin-resistant transformants.
2.5 Sirodesmin PL antibacterial assays
An antibacterial bioassay was carried out essentially as previously described (Elliott et al., 2007). V8 agar cultures (six day old) of transformants, the wild type isolate and ΔsirP mutant (Gardiner et al., 2004) were overlaid with a suspension of Bacillus subtilis (NCTC 8236) in Luria Broth agar. Plates were incubated at 37°C and the presence of zones of clearing around the fungal colony was assessed after 16 h.
2.6 Extraction and analysis of sirodesmin PL and gliotoxin
Filtrates of four and six day old cultures of L. maculans grown in 10% V8 juice were extracted twice with ethyl acetate. Production of sirodesmin PL in triplicate cultures was assessed by High Performance Liquid Chromatography (HPLC) as described by Gardiner et al. (2004), with sirodesmin PL as a standard. Filtrates of 1, 2, 3, 4 and 10 day shaking cultures of A. fumigatus grown at 25°C or 37°C in 10% V8 juice or Czapek Dox or Czapek Dox with 0.5% Yeast or glucose minimal medium (GMM) were extracted twice with ethyl acetate. Production of gliotoxin in triplicate cultures was assessed by HPLC, essentially as described by (Gardiner et al., 2004) and (Gardiner and Howlett, 2005).
2.7 Gene expression analysis
Total RNA was extracted from lyophilised mycelia of L. maculans using TRIzol (Invitrogen), according to manufacturer's instructions, and was DNaseI-treated (Invitrogen) prior to oligo(dT) primed reverse transcription with SuperScriptIII (Invitrogen). The primers used in quantitative Reverse Transcription-PCR (qRT-PCR) experiments to analyse the expression of genes in the sirodesmin PL gene cluster and flanking genes: sirD, sirH, sirP, sirZ, LmMP1, LmPKS1, LmUVI-1h were sirDF and sirDR; sirHF and sirHR; sirPF and sirPR; sirZF and sirZR; MP1F and MP1R; PKS1F and PKS1R and UV1F and UV1R, respectively (Gardiner et al., 2004). Actin was amplified from genomic DNA of L. maculans IBCN 18 using primers act1F and act1R (Gardiner et al., 2004). The primers used to analyse the expression of LmHDX1 and LmZnR were HDX1F and HDX1R and ZnFF and ZnFR, respectively (Table 2). Amplification conditions for these PCR reactions were as described in Gardiner et al.(2004) and annealing temperatures were optimized for each set of primers.
Total RNA was extracted from lyophilised mycelia of A. fumigatus using TRIzol (Invitrogen), according to manufacturer's instructions. Northern blotting, probe preparation and hybridization were performed using standard methods (Sambrook et al., 1989). Blots of A. fumigatus RNA were hybridized with the following fragments, which had been amplified from genomic DNA and labelled with [32P] αdCTP: a 573 bp fragment of L. maculans sirZ (primers sirZ2F and sirZ1R), a 467 bp fragment of P. lilacinoechinulatum PlgliZ (primers PlgliZ3F and PlgliZ3R), a 173 bp fragment of A. fumigatus gliP (primers AfpsF and AfpsR) and a 242 bp fragment of A. fumigatus gliI (primers AfaccsF and AfaccsR) (Table 2).
2.8 Bioinformatic analysis of promoter regions of sirodesmin PL and gliotoxin biosynthetic genes in Leptosphaeria maculans, Aspergillus fumigatus and Penicillium lilacinoechinulatum
Nucleotide sequences 1 kb upstream of the coding region of ETP biosynthetic genes from the three gene clusters were examined for the presence of palindromic motifs, using the MEME motif discovery tool (Bailey and Elkan 1994, 1995). Multiple analyses were performed varying the width of the motifs being sought, and the search was performed on both strands. Other parameters were not changed. For the analysis of sequences in the A. fumigatus cluster (AFUA_6G09630-AFUA_6G09745), the nucleotide and dinucleotide frequencies in 500 bp upstream of 9630 A. fumigatus annotated genes were determined using the fasta-get-markov perl script included in the MEME distribution files. The MEME program was run with these frequencies included in the parameters to calculate the probability of these motifs occurring by chance (Bailey and Elkan, 1994). Including the nucleotide and dinucleotide frequency across all promoters increases the sensitivity of MEME program. The identified motif was processed using the BLOCKS multiple alignment processor and the logo program was used to generate the nucleotide frequency graph. This motif was then used in the MAST program (Bailey and Gribskov, 1998), again including the nucleotide and dinucleotide frequency background file to search for the occurrence of the motif in promoters of the 9630 A. fumigatus genes. All settings were left as default including the e-value cut-off of 10.
3. Results
3.1 Analysis of Zn2(II)Cys6 DNA sequences in Leptosphaeria maculans, Aspergillus fumigatus and Penicillium lilacinoechinulatum
The L. maculans sirZ gene has been previously annotated as having a 1500 bp open reading frame, encoding a 500 amino acid protein (Gardiner et al., 2004). In order to determine intron positions and transcription start and stop positions of this gene, cDNA was subjected to RT- and RACE PCR methods. This analysis confirmed that the sirZ open reading frame is 1500 bp and is interrupted by two introns of 93 bp and 106 bp (Fig. 2). The putative Zn2(II)Cys6 DNA binding domain is at amino acid positions 23-57. The translated sequence shows substantial sequence similarity to A. fumigatus GliZ, particularly in the DNA binding domain (57% identity/72% similarity) (Fig. 2). GliZ, a 454-amino-acid protein, is encoded by a 1413 bp open reading frame, which is not predicted to contain any introns. Similar to SirZ, the putative Zn2(II)Cys6 DNA binding domain is at the N-terminal of the protein (amino acid positions 23-57). The 1350 bp open reading frame of P. lilacinoechinulatum PlgliZ predicted a 449-amino-acid protein without introns with a putative Zn2(II)Cys6 DNA binding domain at amino acid position 21-55. Across the Zn2(II)Cys6 DNA binding domain, PlGliZ shows substantial amino acid similarity to A. fumigatus GliZ (80% identity, 88% similarity), but less to L. maculans SirZ (40% identity, 55% similarity) (Fig. 2).
Fig. 2.

Alignment of the predicted amino acid sequences of Leptosphaeria maculans SirZ (LmSirZ; GenBank accession no. AAS92551), Aspergillus fumigatus GliZ (AfGliZ; GenBank accession no. AAW03310), Penicillium lilacinoechinulatum (PlGliZ; GenBank accession no. EF429247; Patron et al., 2007) and L. maculans ZnR (LmZnR; GenBank accession no. AAO49457.1). LmZnR has a Zn(II)2Cys6 DNA binding domain and is not linked to the sirodesmin PL gene cluster. The black line denotes the putative Zn(II)2Cys6 DNA binding domain. Asterisks indicate conserved cysteine residues in this domain. Positions of introns in LmSirZ are denoted by the triangles. Grey shading indicates two or more similar amino acids, black shading indicates two or more identical amino acids.
3.2 RNA-mediated gene silencing of sirZ
RNA-mediated gene silencing was employed to determine whether the Zn2(II)Cys6 transcription factor encoded by sirZ acts as a pathway-specific transcription factor of sirodesmin PL biosynthetic genes. Wild type Leptosphaeria maculans isolate IBCN 18 was transformed with psirZRNAi. The presence of a single copy of psirZRNAi in the three hygromycin-resistant transformants (sirZRNAi2, sirZRNAi4 and sirZRNAi6) was confirmed by Southern analysis (data not shown). No differences in growth and culture morphology were observed between the transformants and isolate IBCN 18. An antibacterial assay was used as an initial screen to determine if any of the transformants were deficient in sirodesmin PL production (Fig. 3). A L. maculans mutant carrying a deletion in sirP, which encodes the non-ribosomal peptide synthetase essential for sirodesmin PL biosynthesis (Gardiner et al., 2004) was used as a control. The wild type strain inhibited bacterial growth, as did transformant sirZRNAi4, although to a much lesser degree. In contrast, the ΔsirP mutant and transformants sirZRNAi2 and sirZRNAi6 did not inhibit bacterial growth, which indicates a deficiency in sirodesmin PL production. Quantitative Reverse-Transcription PCR (qRT-PCR) was then used to determine if the transcription of sirZ was silenced in these transformants (Fig. 4). Transformants sirZRNAi2 and sirZRNAi6 had less than 5% of the level of transcripts of wild type, whereas transformant sirZRNAi4 had approximately 20%, after four days of growth in 10% V8 Juice. A similar result was seen after six days of growth (data not shown).
Fig. 3.
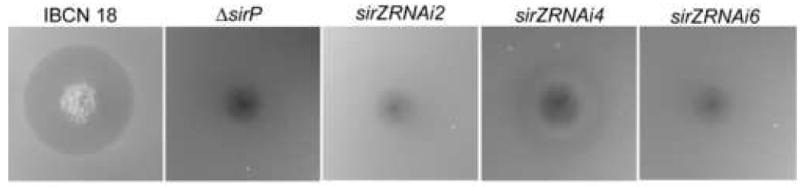
Anti-bacterial assay to detect presence of sirodesmin PL. Six day old colonies of Leptosphaeria maculans wild type isolate IBCN 18, a peptide synthetase mutant, ΔsirP, which is unable to produce sirodesmin PL and psirZRNAi transformants, sirZRNAi2, sirZRNAi4 and sirZRNAi6 growing on 10% Campbell's V8 juice agar, were overlaid with molten agar containing Bacillus subtilis and incubated overnight at 37°C. The wild type isolate produces a ring of clearing, indicative of the production of high levels of sirodesmin PL, whilst ΔsirP, sirZRNAi2 and sirZRNAi6 do not produce rings of clearing. Transformant sirZRNAi4 does produce a ring of clearing, although it is much smaller than that produced by wild type.
Fig. 4.
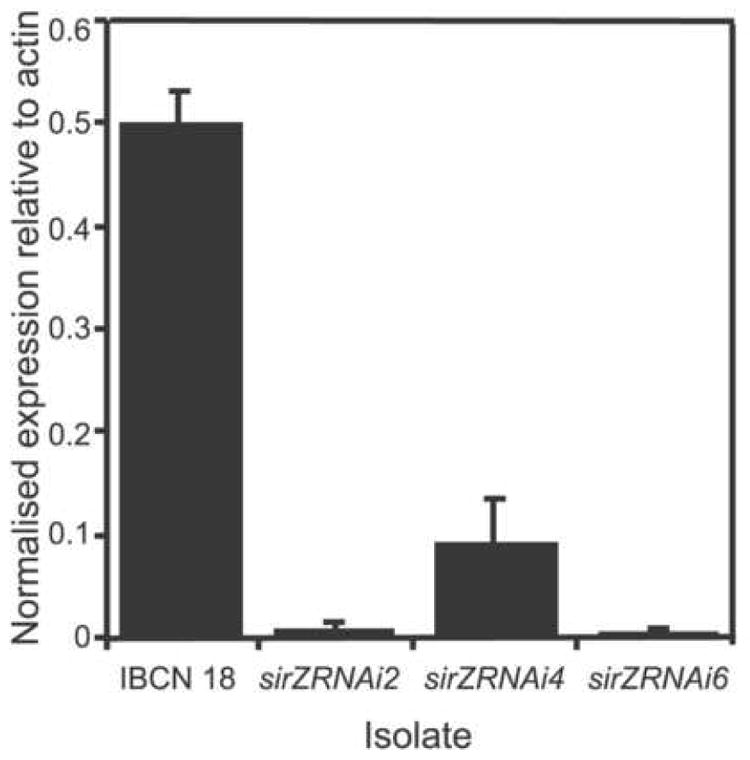
Quantitative Reverse Transcription PCR analysis of the transcription of sirZ in wild type Leptosphaeria maculans (IBCN 18) and sirZ-silenced transformants (sirZRNAi2, sirZRNAi4 and sirZRNAi6). Conidia (106) were inoculated into complete medium (10% V8 juice; 50 mL) and grown at 22°C in the dark, without agitation, for 4 days. Gene expression level is normalised to the expression level of actin. Values are means ± SE of triplicate reactions of three independent biological samples. Levels of sirZ transcripts are markedly reduced in transformants sirZRNAi2, sirZRNAi4 and sirZRNAi6. This experiment was repeated three times and consistent results were seen.
3.3 RNA-mediated silencing of sirZ results in reduced production of sirodesmin PL and decreased transcription of sirodesmin PL biosynthetic genes
Culture filtrates of transformants sirZRNAi2, sirZRNAi4 and sirZRNAi6 grown in 10% V8 juice for four days, were screened for sirodesmin PL production using HPLC. In accordance with the results from the antibacterial assays, transformants sirZRNAi6 and sirZRNAi2 did not produce detectable levels of sirodesmin PL while transformant sirZRNAi4 did produced detectable levels of sirodesmin PL, although at much lower levels than wild type (Fig. 5). To determine if the decreased production of sirodesmin PL in transformant sirZRNAi6 was mediated by altered regulation of sirodesmin PL biosynthetic genes, qRT-PCR was used to assess the transcription levels of some of these clustered genes in this transformant and wild type (Fig. 6). Transcription levels of sirP, sirD and sirH were examined as the products of these genes are thought to act at the beginning and the end of the sirodesmin PL biosynthetic pathway (Gardiner et al., 2004). The latter two genes are also unique to the sirodesmin PL biosynthetic cluster, compared to other ETP clusters (Fig. 1). The transcription of these three genes was significantly reduced in sirZRNAi6 compared to wild-type (Fig. 6).
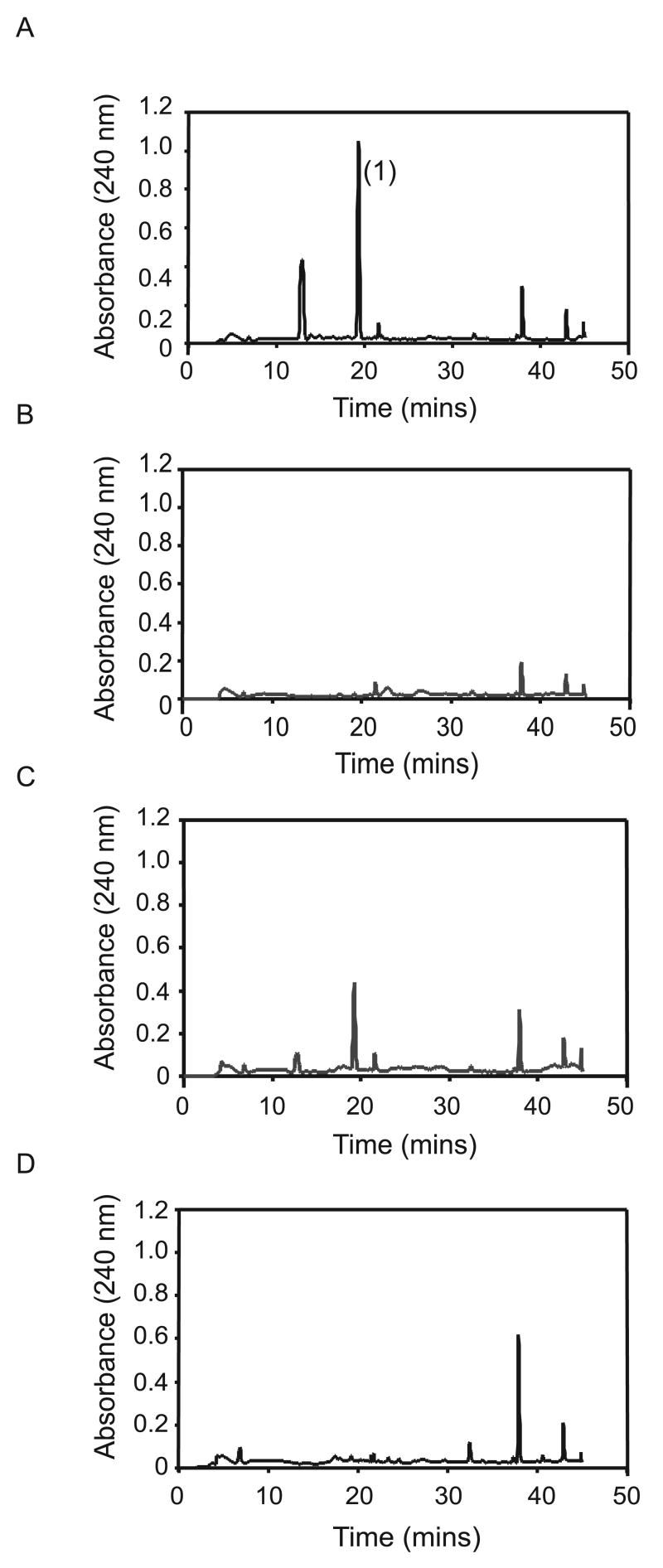
Fig. 5.
HPLC profiles of culture filtrates of wild type Leptosphaeria maculans IBCN 18 (A) and sirZ-silenced transformants, sirZRNAi2 (B), sirZRNAi4 (C) and sirZRNAi6 (D). The cultures used for sirodesmin PL extraction were those used for transcriptional analysis (Fig. 4). The culture filtrate was extracted twice with ethyl acetate and subjected to HPLC as described in Gardiner et al. (2004). Absorbance was measured at 240 nm. The major peak (1) eluting at 18.2 min has identical retention properties to a standard preparation of sirodesmin PL and its amount is reduced in sirZ-silenced transformant, sirZRNAi4 and greatly reduced in sirZ-silenced transformants, sirZRNAi2 and sirZRNAi6.
Fig. 6.
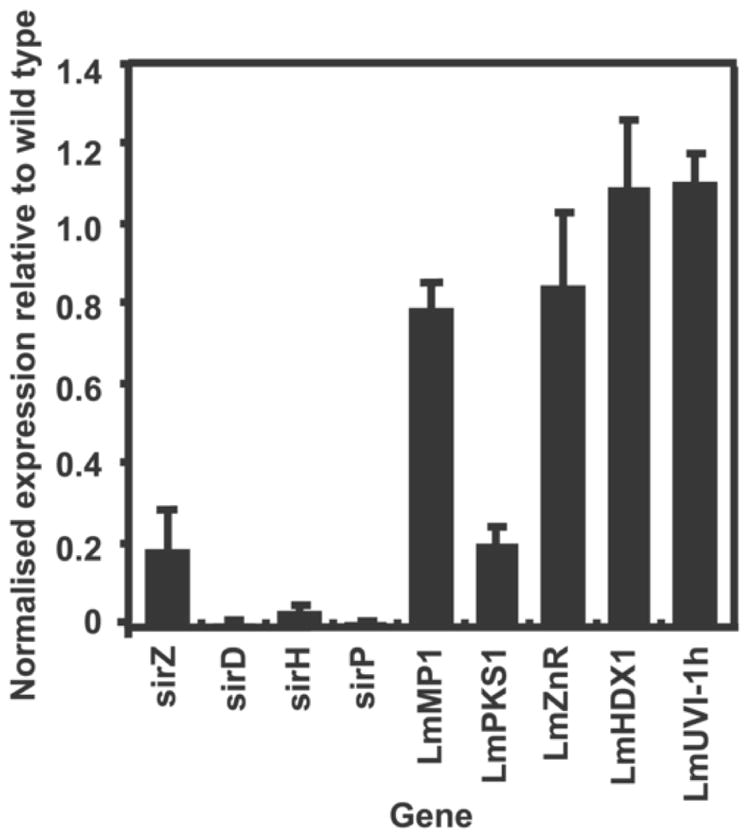
Quantitative Reverse Transcription PCR analysis of the transcription of Leptosphaeria maculans genes in wild type L. maculans and sirZ-silenced transformant, sirZRNAi 6. The cultures used for RNA extraction were those used for the analysis of sirZ transcription (Fig. 4). Gene expression level is relative to actin and normalised to expression level in wild type isolate IBCN 18 cultures grown under the same conditions. Values are means ± SE of triplicate reactions of three independent biological samples. This experiment was repeated three times and consistent results were seen in all experiments. The transcription of sirodesmin PL biosynthetic genes (sirD, sirH, sirP and sirZ) is reduced in sirZRNAi 6 compared to wild type. With the exception of LmPKS, the transcription of genes flanking the cluster (LmHDX1, LmMP1 and LmUV1-1h) is relatively unchanged between sirZRNAi 6 and wild-type. The transcription of LmZnR, a Zn(II)2Cys6 domain containing gene, which is unlinked to the cluster, is also relatively unchanged between sirZRNAi 6 and wild type. This experiment was repeated three times and consistent results were seen.
A previous study showed that genes directly flanking the sirodesmin PL biosynthetic were not co-regulated with genes within the cluster (Gardiner et al., 2004). To analyse whether SirZ is regulating only genes within the cluster, the transcription levels of four genes flanking the cluster (LmHDX1, LmMP1, LmPKS and LmUVI-1h) were measured in sirZRNAi6 and wild-type. With the exclusion of LmPKS, transcription of these genes was largely unchanged in sirZRNAi6 compared to wild type IBCN 18 (Fig. 6). These patterns were consistent in three independent experiments. The expression of a Zn(II)2Cys6 domain-containing gene, LmZnR, which is unlinked to the sirodesmin PL biosynthetic cluster (Idnurm et al., 2003), was also unchanged in sirZRNAi6 compared to wild type.
3.4 Leptosphaeria maculans sirZ and Penicillium lilacinoechinulatum PlgliZ cannot functionally complement an Aspergillus fumigatus ΔgliZ mutant
As a gene encoding a Zn(II)2Cys6 DNA binding protein is conserved in the ETP biosynthetic gene clusters of L. maculans and A. fumigatus and the putative ETP biosynthetic gene cluster of P. lilacinoechinulatum, we were interested to see whether the function of this protein is conserved. To test this, an A. fumigatus ΔgliZ mutant was transformed with pLmsirZ or pPlgliZ, which are plasmids containing genomic sequence of L. maculans sirZ or P. lilacinoechinulatum gliZ, respectively. These plasmids contained approximately 1 kb of promoter and 500 bp terminator sequence. The presence of the entire sirZ and PlgliZ sequence was confirmed by Southern analysis of six hygromycin-resistant transformants, ΔgliZ:LmsirZ1ΔgliZ:LmsirZ2, ΔgliZ:LmsirZ3 and ΔgliZ:PlgliZ1, ΔgliZ:PlgliZ2, ΔgliZ:PlgliZ3, respectively (data not shown). Northern analysis of RNA extracted from 1, 2, 3, 4 and 10 day-old shaking cultures grown at 25°C or 37°C in 10% V8 juice, Czapek Dox, Czapek Dox with 0.5% Yeast or glucose minimal medium (GMM) showed that PlgliZ was highly transcribed in ΔgliZ:PlgliZ3 under all of these conditions. Figure 7 shows the transcription of PlgliZ in this transformant after two days incubation in Czapek Dox medium at 37°C. However, transcription of sirZ was undetected in ΔgliZ:LmsirZ1, ΔgliZ:LmsirZ2 or ΔgliZ:LmsirZ3 under any of the conditions listed above and gliotoxin production was not complemented (data not shown). Despite high transcription levels, PlgliZ failed to complement the gliotoxin-deficiency of A. fumigatus ΔgliZ under the conditions tested (data not shown). Furthermore, PlgliZ was unable to complement the loss of transcription of gliotoxin biosynthetic genes (Fig. 7).
Fig. 7.
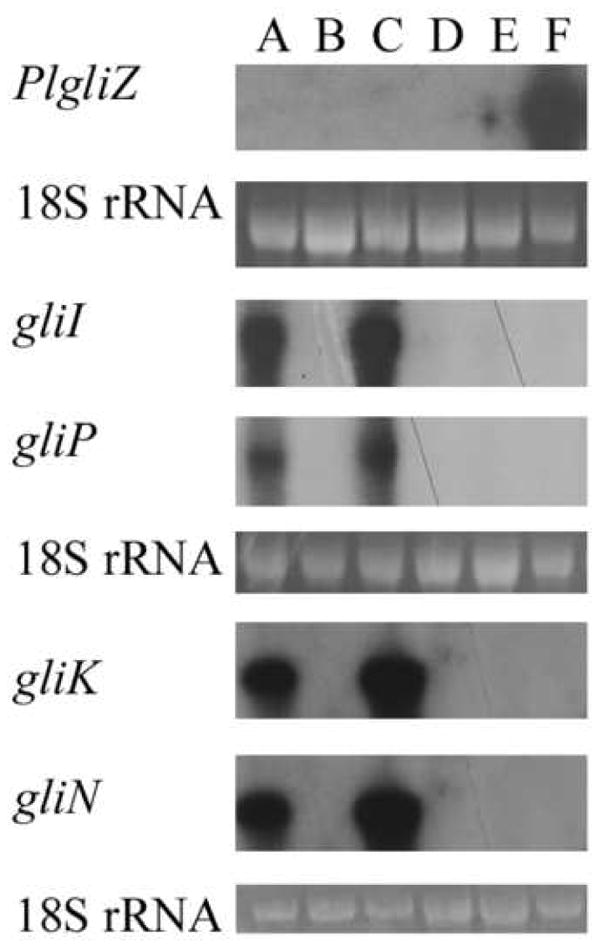
Expression of Penicillium lilacinoechinulatum PlgliZ and gliotoxin biosynthetic genes in isolates of Aspergillus fumigatus. Ethidium bromide-stained 18S rRNA is shown as a control for RNA loading. Total RNA was extracted from shaking cultures grown in Czapek Dox medium at 37°C for two days. RNA from A. fumigatus isolates: A) wild-type (Af293); B) ΔgliZ; C) ΔgliZ:AfgliZ; D) ΔgliZ:PlgliZ1; E) ΔgliZ:PlgliZ2 and F) ΔgliZ:PlgliZ3 was probed with PlgliZ and A. fumigatus gliotoxin biosynthetic genes gliP, gliI, gliK and gliN. PlgliZ is transcribed at a high level in transformant ΔgliZ:PlgliZ3, but not in transformants ΔgliZ:PlgliZ1 and ΔgliZ:PlgliZ2, under these conditions. Although the transcription of these genes was restored when the ΔgliZ mutant was homologously complemented, PlgliZ did not restore transcription in transformant ΔgliZ:PlgliZ3 under these conditions. The same result was found for isolates grown in a range of media.
3.5 Computational analysis of upstream sequences of putative ETP biosynthetic genes in Leptosphaeria maculans, Penicillium lilacinoechinulatum and Aspergillus fumigatus
Zn(II)2Cys6 DNA binding domains commonly bind to palindromic trinucleotides spaced by a variable sequence of defined length. Sequences 1 kb upstream of the predicted start codons of putative ETP biosynthetic genes in L. maculans, P. lilacinoechinulatum and A. fumigatus were searched as a single group for statistically over-represented palindromic binding sites using the MEME motif discovery tool. A motif (consensus TCGGN3CCGA) was consistently identified using different combinations of input sequences (either individual species or as one group of sequence) and motif width parameter (Fig. 8A). This motif was identified in the promoters of sirT, sirP. sirO. sirN, sirP, sirB, sirR, sirJ and sirQ in L. maculans (Fig. 8C), gliI, gliJ, gliP, gliC, gliM, gliG, gliK, gliN, gliF, gliZ and gliT in A. fumigatus (Fig. 8D), and PlgliI, PlgliC, PlgliM, PlgliP, PlgliJ and PlgliZ in P. lilacinoechinulatum (Fig. 8E). It was not present in the promoters of sirZ, sirZ, sirE, sirC, sirS, sirM, sirG, sirH or sirI in L. maculans, gliZ or gliA in A. fumigatus, or PlgliG, PlgliK. PlgliT or PlgliA in P. lilacinoechinulatum. The motif was also absent in the promoters of genes flanking the L. maculans cluster or those flanking the P. lilacinoechinulatum cluster. However, this motif was present in two genes flanking the A. fumigatus gliotoxin biosynthetic cluster, AFUA_6G09745A, which encodes a conserved hypothetical protein and AFUA_6G09610A, which encodes a putative non-ribosomal peptide synthetase (Nierman et al., 2005). Using a motif width of 11 bp, the probability of this element occurring by chance in a random set of sequences having the same nucleotide frequency as the input sequence is 1.6×e-11.
Fig. 8.
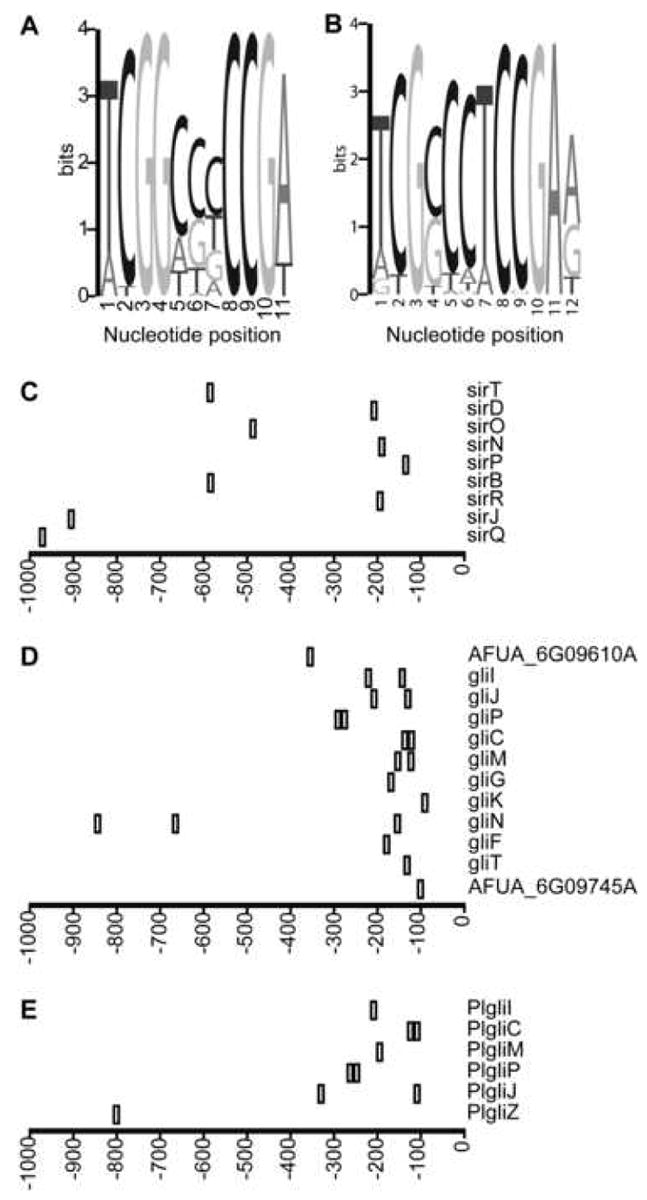
Putative Zn(II)2Cys6 DNA binding motifs in the promoter regions of putative ETP cluster genes. (A) Logo diagram of the predicted palindromic element showing frequency of nucleotides in the statistically overrepresented sequences. (B) Logo diagram of the element predicted using the Aspergillus fumigatus sequences with a nucleotide and dinucleotide frequency background model derived from the upstream regions of all 9630 annotated A. fumigatus genes. Location of statistically significant instances of the element (shown in A) in the upstream regions of Leptosphaeria maculans (C), A. fumigatus (D) and Penicillium lilacinoechinulatum (E) cluster genes. The motif in all of these upstream regions is drawn to scale. The motif was also identified in the promoter regions of two genes flanking the A. fumigatus cluster, AFUA_6G09745A and AFUA_6G09610A. All positions are shown with respect to the translational start (zero).
The availability of the complete genome sequence of A. fumigatus allowed a more robust prediction of overrepresented motifs in the regions upstream of gliotoxin biosynthetic genes. Nucleotide and dinucleotide frequencies in the 500 bp upstream of all 9630 annotated genes were submitted as input into the prediction model for overrepresented sequence motifs in the upstream regions of gliotoxin biosynthetic genes. An essentially identical motif to that predicted without the inclusion of the nucleotide and dinucleotide frequencies, was predicted (Fig. 8B). The major difference between these motifs is the confidence of the prediction of the three nucleotides that are flanked by the indirect repeats. The probability of finding this motif in the region 500 bp upstream of any other gene is 1.7×e-16. Using the MAST program, this motif was found in the upstream regions of 28 of the 9630 genes. Ten of these 28 genes were gliotoxin cluster genes, four of which had multiple copies of the motif (Fig. 8D), confirming that the motif is significantly overrepresented in this cluster compared to the rest of the genome
4. Discussion
Little is known about gene clusters involved in secondary metabolism in Leptosphaeria maculans. To date, only two clusters of genes have been characterised, a 38 kb cluster of unknown function (Idnurm et al., 2003) and the sirodesmin PL biosynthetic gene cluster (Gardiner et al., 2004). Both clusters contain a gene that encodes a Zn(II)2Cys6 DNA binding protein. Five genes in the former cluster showed homology to genes involved in antibiotic or toxin production in bacteria and/or fungi, which prompted the suggestion that this cluster may be involved in secondary metabolism. However, the disruption of a gene encoding a Zn(II)2Cys6 protein (znr1) and a gene with a best match to a monomodular non-ribosomal peptide synthetase (maa1), did not affect the production of known secondary metabolites in L. maculans (Idnurm et al., 2003). The disruption of these two genes, like that of other L. maculans genes, was very difficult due to the large amount of homologous DNA required for targeted integration and the high number of transformants required to be screened. The completion of the L. maculans genome sequence project is likely to uncover new genes, which would also be predicted to have a role in secondary metabolism. However, such predictions obviously need to be validated via genetic analysis. The low efficiency of gene disruption limits the development of high throughout analysis of gene function in this fungus. To overcome this limitation, other methods including random insertional mutagenesis followed by screening for the desired phenotypic trait (Elliott and Howlett, 2006) and RNAi-mediated gene silencing (this study and Fudal et al. (2007)) are being exploited. Although RNAi-mediated silencing does not result in complete eradication of the targeted transcript, partial silencing of genes has been shown to cause mutant phenotypes (Cardoza et al., 2007; Fitzgerald et al., 2004). The ability to correlate the degree of gene silencing with the extent of the phenotype is a tool for validating the role of the gene silencing in the generation of the phenotype. Indeed, in the current study, the level of sirZ expression in the silenced mutants correlated with the level of sirodesmin PL production.
Due to its sequence similarity to other transcriptional regulators and its presence within the sirodesmin PL biosynthetic gene cluster, sirZ was an ideal candidate for the pathway-specific regulator of this gene cluster. RNA-mediated silencing of sirZ confirmed the role of this gene in the regulation of sirodesmin PL production via the transcriptional control of sirodesmin PL biosynthetic genes. The undetectable production of sirodesmin PL when levels of sirZ transcripts are severely reduced suggests that the regulation of sirodesmin PL biosynthetic genes is very specific and cannot be compensated by other Zn(II)2Cys6 DNA binding proteins encoded within the genome.
The silencing of sirZ transcription also provided an opportunity to further define the boundaries of the sirodesmin PL biosynthetic gene cluster. Genes flanking the cluster had been defined due to their independent expression from other cluster genes and their proposed functions, based on sequence similarity to characterised genes, which were thought to be unnecessary for the biosynthesis of sirodesmin PL (Gardiner et al., 2004). Indeed, the silencing of sirZ did not affect the expression of three of the four flanking genes tested, suggesting that these genes probably do not form part of the gene cluster. However, the expression of one flanking gene, LmPKS1, was reduced by the silencing of sirZ. This gene has a best BLAST match to A. parasiticus pksL1, which encodes a polyketide synthetase involved in aflatoxin biosynthesis (Feng et al., 1995). Our findings are inconsistent with the lack of co-ordinated expression of LmPKS1 with other sir genes (Gardiner et al., 2004). It is possible that SirZ is involved in the regulation of genes that are not involved in the biosynthesis of sirodesmin PL. AflR was recently shown to affect transcription of additional genes outside the aflatoxin biosynthetic gene cluster in Aspergillus parasiticus (Price et al., 2006).
Heterologous complementation is an alternative method to gene disruption that allows gene function to be determined. We attempted to examine the function of sirZ and PlgliZ by transforming a deletion mutant of A. fumigatus gliZ. Attempts to complement this mutant with L. maculans sirZ were unsuccessful. Despite testing a range of conditions, sirZ transcription was never detected in any of the transformants. The expression of sirZ might require activation by a protein absent in A. fumigatus or the 1 kb of native promoter may have been insufficient to induce expression. In addition to L. maculans sirZ, we also attempted to cross-complement A. fumigatus ΔgliZ with P. lilacinoechinulatum PlgliZ. As mutational analysis of the ETP-like cluster in P. lilacinoechinulatum has not been performed, successful complementation of A. fumigatus ΔgliZ by PlgliZ would provide evidence that this cluster was responsible for gliotoxin production. However, despite PlgliZ being transcribed in one transformant under all conditions tested, no gliotoxin was detected in this transformant. Although heterologous complementation of regulators of secondary metabolite biosynthesis has been successful between species of a particular genus (Chang et al., 1993; Yu et al., 1996), to our knowledge, it has not been achieved between species belonging to a different genus. The sequence similarity between PlgliZ and gliZ may indeed be too low to afford heterologous complementation. Also PlGliZ may not play a homologous role to GliZ in the regulation of gliotoxin biosynthesis in P. lilacinoechinulatum. To determine whether PlGliZ regulates gliotoxin biosynthesis, mutational studies need to be carried out in this fungus.
Potential binding sites in the promoters of cluster genes were predicted for L. maculans SirZ, A. fumigatus GliZ and P. lilacinoechinulatum PlGliZ by seeking statistically significantly overrepresented palindromic binding sites. Although the identified sites were similar to those commonly bound by Zn(II)2Cys6 DNA binding proteins, the presence of these sites was not always consistent with the regulation pattern of the genes. For example, the palindromic element (consensus TCGGN3CCGA) was found in the promoters of sirT, sirD, sirP, sirB, sirJ, sirQ, sirR, sirO and sirN but not other sir genes (sirH, sirI, sirG, sirC, sirM, sirE, sirS, sirZ or sirA), despite the fact these latter sir genes show similar transcription patterns to the former genes (Gardiner et al., 2004). Similarly in A. fumigatus and P. lilacinoechinulatum, not all promoters were predicted to have the palindromic element, despite evidence in A. fumigatus that the genes are tightly co-regulated (Gardiner and Howlett, 2005). However this is not unexpected as the DNA binding capacity of transcriptional regulator proteins typically deviates from a core consensus, and is likely to be dependent on other sequences and proteins present on the promoter. For example, in trichothecene-producing Fusarium sporotrichioides, the canonical TRI6 binding site is absent from the promoter of TRI4 which encodes the second through fifth steps in trichothecene biosynthesis (Hohn et al., 1999; McCormick et al., 2006). Analysis of TRI6 binding in the TRI4 promoter indicates some flexibility in the recognition sequence (Hohn et al., 1999). Also, it has been shown that AflR may actually recognize several binding sites with fairly significant deviation from the core sequence (Ehrlich et al., 1999).
Although the palindromic element was not identified in the promoter regions of genes flanking the L. maculans sirodesmin PL and P. lilacinoechinulatum putative gliotoxin biosynthetic cluster, this element was present in two genes flanking the A. fumigatus gliotoxin biosynthetic cluster, AFUA_6G09745A, which encodes a conserved hypothetical protein and AFUA_6G09610A, which encodes a putative non-ribosomal peptide synthetase (Nierman et al., 2005). However, it is not known if these sites are biologically relevant. Gel Shift binding assays with oligonucleotides encoding modification of the putative binding sites and purified SirZ, GliZ and PlGliZ will be necessary to confirm the biological relevance of all of these putative binding sites.
This study demonstrates that the biosynthesis of sirodesmin PL in Leptosphaeria maculans is regulated by the Zn(II)2Cys6 DNA-binding protein encoded by sirZ. SirZ mediates its regulatory effect by controlling the transcription of clustered sirodesmin PL biosynthetic genes. SirZ shows sequence similarity to GliZ, the regulator of gliotoxin biosynthesis in A. fumigatus and PlGliZ, the putative regulator of gliotoxin biosynthesis in P. lilacinechinulatum. The role of PlgliZ in the regulation of gliotoxin biosynthesis could not be confirmed via the cross complementation of an A. fumigatus gliotoxin-deficient mutant, ΔgliZ, despite being heterologously transcribed. Computational analysis of promoter regions of ETP biosynthetic genes in L. maculans, P. lilacinoechinulatum and A. fumigatus has led to the prediction of binding sites for the regulatory proteins SirZ, PlGliZ and GliZ, respectively. This paves the way for functional analysis of these binding sequences in relation to pathway-specific regulation of secondary metabolite biosynthesis by Zn2(II)Cys6 DNA binding proteins.
Acknowledgments
We thank the Australian Grains Research and Development Corporation for a Grains Research Scholar award to EMF, and the National Institutes of Health 1 R01 Al065728-01 for research funding to NPK. We thank Dr Jin-Woo Bok for the A. fumigatus ΔgliZ mutant, Dr. Soledade Pedras for the gift of sirodesmin PL, Dr William Nierman and Dr Natalie Fedorova for access to the upstream sequences of 9630 annotated genes of A. fumigatus, and Dr Adrienne Sexton for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander NJ, Hohn TH, McCormick SP. The TRI11 gene of Fusarium sporotrichiodes encodes a cytochrome P-450 monooxygenase required for C-15 hydroxylation in trichothecene biosynthesis. Appl Environ Microbiol. 1998;64:221–225. doi: 10.1128/aem.64.1.221-225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In: Altman R, Brutlag D, Karp P, Lathrop R, Searls D, editors. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology; Menlo Park: AAAI Press; 1994. pp. 28–36. [PubMed] [Google Scholar]
- Bailey TL, Elkan C. The value of prior knowledge in discovering motifs with MEME. Proc Int Conf Intell Syst Mol Biol. 1995;3:21–29. [PubMed] [Google Scholar]
- Bailey TL, Gribskov M. Combining evidence using p-values: application to sequence homology searches. Bioinformatics. 1998;14:48–54. doi: 10.1093/bioinformatics/14.1.48. [DOI] [PubMed] [Google Scholar]
- Bok JW, Balajee SA, Marr KA, Andes D, Nielsen KF, Frisvad JC, Keller NP. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot Cell. 2005;4:1574–1582. doi: 10.1128/EC.4.9.1574-1582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, Chung D, Balajee SA, Marr KA, Andes D, Nielsen KF, Frisvad JC, Kirby KA, Keller NP. GliZ, a transcriptional regulator of gliotoxin biosynthesis, contributes to Aspergillus fumigatus virulence. Infect Immun. 2006;74:6761–6768. doi: 10.1128/IAI.00780-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DW, Butchko RA, Busman M, Proctor RH. The Fusarium verticillioides FUM gene cluster encodes a Zn(II)2Cys6 protein that affects FUM gene expression and fumonisin production. Eukaryot Cell. 2007;6:1210–1218. doi: 10.1128/EC.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu'Lock JD, Leigh C. Biosynthesis of gliotoxin. J Chem Soc Chem Commun. 1975:628–629. [Google Scholar]
- Cardoza RE, Hermosa MR, Vizcaino JA, Gonzalez F, Llobell A, Monte E, Gutierrez S. Partial silencing of a hydroxy-methylglutaryl-CoA reductase-encoding gene in Trichoderma harzianum CECT 2413 results in a lower level of resistance to lovastatin and lower antifungal activity. Fungal Genet Biol. 2007;44:269–283. doi: 10.1016/j.fgb.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Chang PK, Cary JW, Bhatnagar D, Cleveland TE, Bennett JW, Linz JE, Woloshuk CP, Payne GA. Cloning of the Aspergillus parasiticus apa-2 gene associated with the regulation of aflatoxin biosynthesis. Appl Environ Microbiol. 1993;59:3273–3279. doi: 10.1128/aem.59.10.3273-3279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang PK, Ehrlich KC, Yu J, Bhatnagar D, Cleveland TE. Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA-binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl Environ Microbiol. 1995;61:2372–2377. doi: 10.1128/aem.61.6.2372-2377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lee MH, Daub ME, Chung KR. Molecular analysis of the cercosporin biosynthetic gene cluster in Cercospora nicotianae. Mol Microbiol. 2007;64:755–770. doi: 10.1111/j.1365-2958.2007.05689.x. [DOI] [PubMed] [Google Scholar]
- Cramer RA, Jr, Gamcsik MP, Brooking RM, Najvar LK, Kirkpatrick WR, Patterson TF, Balibar CJ, Graybill JR, Perfect JR, Abraham SN, Steinbach WJ. Disruption of a nonribosomal peptide synthetase in Aspergillus fumigatus eliminates gliotoxin production. Eukaryot Cell. 2006;5:972–980. doi: 10.1128/EC.00049-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich KC, Montalbano BG, Cary JW. Binding of the C6-zinc cluster protein, AFLR, to the promoters of aflatoxin pathway biosynthesis genes in Aspergillus parasiticus. Gene. 1999;230:249–257. doi: 10.1016/s0378-1119(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Elliott CE, Gardiner DM, Thomas GD, Cozijnsen AJ, Van De Wouw AP, Howlett BJ. Production of the toxin sirodesmin PL by Leptosphaeria maculans during infection of Brassica napus. Mol Plant Pathol. 2007 doi: 10.1111/J.1364-3703.2007.00433.X. In Press. [DOI] [PubMed] [Google Scholar]
- Elliott CE, Howlett BJ. Overexpression of a 3-ketoacyl-CoA thiolase in Leptosphaeria maculans causes reduced pathogenicity on Brassica napus. Mol Plant Microbe Interact. 2006;19:588–596. doi: 10.1094/MPMI-19-0588. [DOI] [PubMed] [Google Scholar]
- Feng B, Friedlin E, Marzluf GA. Nuclear DNA-binding proteins which recognize the intergenic control region of penicillin biosynthetic genes. Curr Genet. 1995;27:351–358. doi: 10.1007/BF00352104. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Keller NP, Adams TH. Sequence-specific binding by Aspergillus nidulans AflR, a C6 zinc cluster protein regulating mycotoxin biosynthesis. Mol Microbiol. 1998;28:1355–1365. doi: 10.1046/j.1365-2958.1998.00907.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald A, Van Kan JA, Plummer KM. Simultaneous silencing of multiple genes in the apple scab fungus, Venturia inaequalis, by expression of RNA with chimeric inverted repeats. Fungal Genet Biol. 2004;41:963–971. doi: 10.1016/j.fgb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Fudal I, Ross S, Gout L, Blaise F, Kuhn ML, Eckert MR, Cattolico L, Bernard-Samain S, Balesdent MH, Rouxel T. Heterochromatin-like regions as ecological niches for avirulence genes in the Leptosphaeria maculans genome: map-based cloning of AvrLm6. Mol Plant Microbe Interact. 2007;20:459–470. doi: 10.1094/MPMI-20-4-0459. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Cozijnsen AJ, Wilson LM, Pedras MS, Howlett BJ. The sirodesmin biosynthetic gene cluster of the plant pathogenic fungus Leptosphaeria maculans. Mol Microbiol. 2004;53:1307–1318. doi: 10.1111/j.1365-2958.2004.04215.x. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Howlett BJ. Negative selection using thymidine kinase increases efficiency of recovery of transformants with targeted genes in filamentous fungus Leptosphaeria maculans. Curr Genet. 2004;45:249–255. doi: 10.1007/s00294-004-0488-6. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Howlett BJ. Bioinformatic and expression analysis of the putative gliotoxin biosynthetic gene cluster of Aspergillus fumigatus. FEMS Microbiol Lett. 2005;248:241–248. doi: 10.1016/j.femsle.2005.05.046. [DOI] [PubMed] [Google Scholar]
- Gardiner DM, Waring P, Howlett BJ. The epipolythiodioxopiperazine (ETP) class of fungal toxins: distribution, mode of action, functions and biosynthesis. Microbiology. 2005;151:1021–1032. doi: 10.1099/mic.0.27847-0. [DOI] [PubMed] [Google Scholar]
- Helliwell C, Waterhouse P. Constructs and methods for high-throughput gene silencing in plants. Methods. 2003;30:289–295. doi: 10.1016/s1046-2023(03)00036-7. [DOI] [PubMed] [Google Scholar]
- Hohn TM, Krishna R, Proctor RH. Characterization of a transcriptional activator controlling trichothecene toxin biosynthesis. Fungal Genet Biol. 1999;26:224–235. doi: 10.1006/fgbi.1999.1122. [DOI] [PubMed] [Google Scholar]
- Idnurm A, Taylor JL, Pedras M, Howlett BJ. Small scale functional genomics of the blackleg fungus, Leptosphaeria maculans: analysis of a 38 kb region. Austral Plant Path. 2003;32:511–519. [Google Scholar]
- Keller NP, Hohn TM. Metabolic pathway gene clusters in filamentous fungi. Fungal Genet Biol. 1997;21:17–29. [PubMed] [Google Scholar]
- Kim JE, Jin J, Kim H, Kim JC, Yun SH, Lee YW. GIP2, a putative transcription factor that regulates the aurofusarin biosynthetic gene cluster in Gibberella zeae. Appl Environ Microbiol. 2006;72:1645–1652. doi: 10.1128/AEM.72.2.1645-1652.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfahl C, Heinekamp T, Geginat G, Ruppert T, Hartl A, Hof H, Brakhage AA. Deletion of the gliP gene of Aspergillus fumigatus results in loss of gliotoxin production but has no effect on virulence of the fungus in a low-dose mouse infection model. Mol Microbiol. 2006;62:292–302. doi: 10.1111/j.1365-2958.2006.05373.x. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Wiederhold NP, Lionakis MS, Prince RA, Kontoyiannis DP. Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center. J Clin Microbiol. 2005;43:6120–6122. doi: 10.1128/JCM.43.12.6120-6122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick SP, Alexander NJ, Proctor RH. Fusarium Tri4 encodes a multifunctional oxygenase required for trichothecene biosynthesis. Can J Microbiol. 2006;52:636–642. doi: 10.1139/w06-011. [DOI] [PubMed] [Google Scholar]
- McDonald T, Brown D, Keller NP, Hammond TM. RNA silencing of mycotoxin production in Aspergillus and Fusarium species. Mol Plant Microbe Interact. 2005;18:539–545. doi: 10.1094/MPMI-18-0539. [DOI] [PubMed] [Google Scholar]
- Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C, Bennett J, Bowyer P, Chen D, Collins M, Coulsen R, Davies R, Dyer PS, Farman M, Fedorova N, Fedorova N, Feldblyum TV, Fischer R, Fosker N, Fraser A, Garcia JL, Garcia MJ, Goble A, Goldman GH, Gomi K, Griffith-Jones S, Gwilliam R, Haas B, Haas H, Harris D, Horiuchi H, Huang J, Humphray S, Jimenez J, Keller N, Khouri H, Kitamoto K, Kobayashi T, Konzack S, Kulkarni R, Kumagai T, Lafton A, Latge JP, Li W, Lord A, Lu C, Majoros WH, May GS, Miller BL, Mohamoud Y, Molina M, Monod M, Mouyna I, Mulligan S, Murphy L, O'Neil S, Paulsen I, Penalva MA, Pertea M, Price C, Pritchard BL, Quail MA, Rabbinowitsch E, Rawlins N, Rajandream MA, Reichard U, Renauld H, Robson GD, Rodriguez de Cordoba S, Rodriguez-Pena JM, Ronning CM, Rutter S, Salzberg SL, Sanchez M, Sanchez-Ferrero JC, Saunders D, Seeger K, Squares R, Squares S, Takeuchi M, Tekaia F, Turner G, Vazquez de Aldana CR, Weidman J, White O, Woodward J, Yu JH, Fraser C, Galagan JE, Asai K, Machida M, Hall N, Barrell B, Denning DW. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2006;438:1151–1156. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- Patron NJ, Waller RF, Cozijnsen AJ, Straney DC, Gardiner DM, Nierman WC, Howlett BJ. Origin and distribution of epipolythiodioxopiperazine (ETP) gene clusters in filamentous ascomycetes. BMC Evolut Biol. 2007;7:174. doi: 10.1186/1471-2148-7-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price MS, Yu J, Nierman WC, Kim HS, Pritchard B, Jacobus CA, Bhatnagar D, Cleveland TE, Payne GA. The aflatoxin pathway regulator AflR induces gene transcription inside and outside of the aflatoxin biosynthetic cluster. FEMS Microbiol Lett. 2006;255:275–279. doi: 10.1111/j.1574-6968.2005.00084.x. [DOI] [PubMed] [Google Scholar]
- Rouxel T, Chupeau Y, Fritz R, Kollmann A, Bousquet JF. Biological effects of sirodesmin PL, a phytotoxin produced by Leptosphaeria maculans. Plant Science. 1988;57:45–53. [Google Scholar]
- Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1989. [Google Scholar]
- Schumann J, Hertweck C. Molecular Basis of cytochalasan biosynthesis in fungi: gene cluster analysis and evidence for the involvement of a PKS-NRPS hybrid synthase by RNA silencing. J Am Chem Soc. 2007;129:9564–9565. doi: 10.1021/ja072884t. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Keller NP. Genetic involvement of a cAMP-dependent protein kinase in a G protein signaling pathway regulating morphological and chemical transitions in Aspergillus nidulans. Genetics. 2001;157:591–600. doi: 10.1093/genetics/157.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd RB, Andrianopoulos A. Evolution of a fungal regulatory gene family: the Zn(II)2Cys6 binuclear cluster DNA binding motif. Fungal Genet Biol. 1997;21:388–405. doi: 10.1006/fgbi.1997.0993. [DOI] [PubMed] [Google Scholar]
- Waring P, Eichner R, Tiwari-Palni U, Mullbacher A. Gliotoxin-E: a new biologically active epipolythiodioxopiperazine isolated from Penicillium terlikowski. Aust J Chem. 1987;40:991–997. [Google Scholar]
- Wilhite SE, Lumsden RD, Straney DC. Mutational analysis of gliotoxin production by the biocontrol fungus Gliocladium virens in relation to suppression of pythium damping-off. Phytopathology. 1994;84:816–821. [Google Scholar]
- Woloshuk CP, Foutz KR, Brewer JF, Bhatnagar D, Cleveland TE, Payne GA. Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl Environ Microbiol. 1994;60:2408–2414. doi: 10.1128/aem.60.7.2408-2414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JH, Butchko RA, Fernandes M, Keller NP, Leonard TJ, Adams TH. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr Genet. 1996;29:549–555. doi: 10.1007/BF02426959. [DOI] [PubMed] [Google Scholar]