Abstract
High dietary sodium intake is a risk factor for hypertension, and heart rate variability (HRV) is decreased in hypertension. Effects of dietary sodium intake on HRV of normotensive persons have not, however, been investigated to date. The present study examined effects of low and high sodium diets on blood pressure, heart rate, and HRV in 36 healthy, normotensive women, ages 40–70. Each was placed on a low sodium diet for six days followed by a high sodium diet for six days. High salt diet increased mean systolic blood pressure, decreased heart rate, and increased high frequency HRV (HF). Cardiac vagal tone, estimated at baseline from heart period and a time domain estimate of respiratory sinus arrhythmia, was higher in salt-sensitive than salt-insensitive subjects. The finding of increased vagal tone in normotensive persons on high salt intake indicates that dietary sodium status should be considered in behavioral studies of HRV.
Index terms: blood pressure, heart rate variability, sodium
Behavioral interactions can influence the development of cardiovascular disease via effects on autonomic regulation of the heart. Heart rate variability (HRV) is a noninvasive window into autonomic control of cardiac function, and can be analyzed via time and frequency domain measures (Task Force of the European Society, 1996). Power spectral density methodology (Kay & Marple, 1981) identifies periodic components of HRV and estimates their frequency and power. The frequency domain includes a low frequency component (LF), which reflects a combination of sympathetic and parasympathetic influences, and a high frequency component (HF), which reflects parasympathetic influence (Berntson, Cacioppo & Quigley 1993). An estimate of sympathovagal balance is derived from the ratio of low frequency to high frequency power (LF/HF).
Some forms of cardiovascular disease, including hypertension are characterized by decreased HRV (Mortara, et al., 1994; Piccirillo, et al. 1996; Stein & Kleiger, 1999; Virtanen, Jula, Kuusela, Helenius & Voipio-Pulkki, 2003; Lucini, De Vede, Parati & Pagani, 2005). Job strain and low decision latitude have also been associated with decreased HF (e.g. Gallo, Bogart, Vranceanu & Wait, 2004; Collins, Karasek & Costas, 2005).
Several studies have shown that behavioral procedures that increase blood pressure and heart rate acutely also alter HRV consistent with decreases in parasympathetic activity (Madden & Savard, 1995; Reims et al, 2005; Guasti et al, 2005). In addition, decreases in HF of postmenopausal women during a speech preparation task were associated with greater calcification of coronary arteries over the succeeding three-years (Gianaros et al, 2005).
A few studies have examined stress-induced changes in HRV in salt-sensitive (SS) and salt-insensitive (SI) normotensive humans. Some (e.g. Deter et al, 2001) though not all (Falkner & Kushner, 1990) found that mental stress generated larger increases in blood pressure and heart rate in SS than SI subjects. These studies also showed that salt-sensitivity was associated with greater anxiety and irritability (Deter, et al., 2006) and reduced vagal activity in response to mental stress (Buchholz et al 2003). The potential importance of the environment in cardiovascular response to salt-sensitivity was investigated in a study with salt-loaded medical students, who showed larger increases in resting blood pressure when the diet was administered before final exams than during a holiday rest period (Haythornthwaite, Pratley & Anderson, 1992).
To date, however, the effects of sodium loading on HRV in normotensive humans have not been investigated. Since blood pressure response to dietary sodium is a risk factor for the development of hypertension (Weinberger, 2004), identification of autonomic differences between SS and SI normotensives could point to an underlying condition that potentiates the development of chronic hypertension.
The present study assessed the effects of changes in dietary sodium intake on HRV in normotensive persons. Persons over age 40 were studied because older persons have a greater prevalence of salt-sensitivity (Weinberger & Fineberg, 1991). Women only were studied due to gender differences in resting autonomic activity (Evans, Ziegler, & Patwardham, 2001). In the present study, each subject was maintained on a low sodium diet for six days followed by a high sodium diet for six days with 24-hour ambulatory blood pressure monitoring after each diet period. HRV was determined on the last day of each diet via frequency domain indices. It was hypothesized that dietary sodium loading would decrease heart rate and increase HRV indices of parasympathetic activity in response to the accompanying increases in intravascular volume.
METHODS
Participants
Sixty women aged 40–70 were recruited from the surrounding community through local advertisements. After medical examination, 38 (63%) were accepted into and completed the study. Two of the 38 were subsequently excluded from the data analysis when respiratory rate indicated hyperventilatory breathing in the clinic setting. All participants were free of respiratory or cardiovascular disease, were non-smokers, and were not taking any medications that would interfere with the study procedures. Since racial differences in renal regulation of sodium and blood pressure salt sensitivity have been reported, the study was restricted to Caucasians (Weinberger, 2004). The protocol was approved by the Institutional Review Board of the Medstar Research Institute.
Procedures
Potential participants were screened briefly over the telephone for medical history. Qualifying participants were invited to the NIA ASTRA Clinical Research Unit where the study coordinator explained the purpose and methods of the study and obtained informed consent. Medical personnel performed a physical examination, a comprehensive medical history, electrocardiogram (EKG) and obtained blood and urine samples to ensure that the participants met the study entry criteria.
Each qualifying subject participated in a 12-day experiment consisting of six days on a low sodium diet followed immediately by six days on a high sodium diet, similar to those in previous studies laboratories (Sullivan, Prewitt, Ratts, Josephs, & Connor 1988; Anderson et al, 1998; Coruzzi, et al, 2005). Participants were given low sodium-low potassium meals in insulated containers, and provided with dietary logs to record food intake to augment adherence to the diet. During the low sodium diet, participants ingested one mmol/kg sodium (60 mmol) and one mmol/kg potassium (60 mmol). During the high sodium diet, participants ingested four mmol/kg sodium/day (60 mmol) and one mmol/kg potassium (60 mmol) each day. Table 1 shows the macronutrient content and mineral composition of the low and high salt diets. During the high salt diet, participants ingested sodium tablets to supplement sodium intake (Buchholz et al 2003).
Table 1.
Mean and standard deviations of macronutrient and mineral composition of the low salt and high salt diets for 36 normotensive women.
| Low Salt | High Salt | |
|---|---|---|
| Kilocalories (kcal) | 1657 ± 344 | 1640 ± 339 |
| Protein (gm) | 73 ± 6 | 67 ± 10 |
| Carbohydrate (gm) | 198 ± 43 | 250 ± 33 |
| Sodium (mmol) | 58 ± 8 | 154 ± 12 |
| Potassium (mmol) | 59 ± 6 | 62 ± 5 |
| Calcium (mmol) | 16 ± 1 | 14 ± 1 |
| Magnesium (mmol) | 10 ± 1 | 9 ± 1 |
Participants were permitted one caffeinated beverage per day. The study coordinator telephoned each participant during the six-day interval to review food diaries in order to encourage dietary compliance. At the end of each diet period, each subject collected urine for 24 hr, which was kept in an insulated cooler, and returned to the Clinical Research Unit within 24 hr for measurement of sodium and potassium excretion. Sodium and potassium excretion was evaluated to ensure that the participants complied with the dietary regimens.
Physiological Measures
Preceding and following each six-day diet period, breathing parameters, blood pressure, and heart rate were monitored during 25-minute sessions of seated rest in a recliner chair in the Research Unit. Breathing pattern was monitored via the LifeShirt™ (Vivometrics, Ventura, CA), which contains sinusoidal wires sewn into an elasticized vest that expanded with each breath, and provided tidal volume as well as breathing frequency on a breath-to-breath basis. EKG electrodes were taped to the participant’s chest and abdomen and connected to a multi-channel ambulatory microcomputer that recorded and stored data onto compact flashcards. The LifeShirt™ measurement of tidal volume was calibrated before each session by exhaling a fixed volume of air into an inflatable bag.
Blood pressure was obtained in the clinic setting and for 24 hr in the natural environment via an inflatable arm cuff connected to an automated oscillometric device (Spacelabs, model 90207, Redmond, WA). Blood pressure was measured five times during each 25-min clinic session. At baseline and following each six-day diet period, blood pressure was recorded once per hour for 24 hr during ambulatory activity in the natural environment.
Data Analysis
Salt-sensitivity was determined from the 24-hr blood pressure measurements on low and high salt diets. Those participants that showed a systolic blood pressure increase of 5 mmHg or greater in response to high salt diet were classified as salt-sensitive.
HRV was determined from an EKG obtained during 10-min seated rest at each visit. The EKG was downloaded into Vivologic software (Vivometrics, Ventura, CA) and visually inspected on a Microsoft Excel ® worksheet to exclude outliers (i.e. two standard deviations from the session mean). The data were then entered into Kubios HRV Analysis software (MATLAB, version 2 beta, Kuopio, Finland). A piecewise cubic spline interpolation method was applied before calculating the frequency domain measures. For baseline and after six-days on the low and high sodium diets, the low (LF range: 0.04–0.15 Hz) and high (HF range: 0.15–0.40 Hz) frequency components were calculated by parametric autoregressive modeling (Oppenheim & Schafe, 1975). The natural logarithms of LF and HF were analyzed statistically.
General linear models using repeated measures were created to determine the significance of the differences between diet conditions and salt-sensitivity status for blood pressure, heart rate, LF, HF and LF/HF. The significance of the differences between conditions in 24-hr urine volume and electrolyte excretion was determined via two-tailed t-test. Mauchly’s test for sphericity was performed with each repeated measures analysis to show that the necessary assumptions were not violated.
Statistics were performed using SSPS, Version 11 (SPSS Inc., Chicago, Illinois) and SAS, version 9.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Baseline characteristics
Table 2 shows means and standard deviations of physiological measures for SS and SI subgroups at baseline, and after low and high salt diet. Systolic blood pressure at baseline was higher (t = 3.20; p < 0.003), and respiratory rate was lower (t = 2.89; p < 0.007) in SS than SI subjects. In addition HF was marginally lower in SS (t = 1.98; p < 0.056) than SI subjects, while LF/HF was marginally higher (t = 1.90; p < 0.07).
Table 2.
Means and standard deviations of systolic (SBP) and diastolic (DBP) blood pressure, heart rate (HR), breaths per minute (Br/M), tidal volume (TV), minute ventilation (MV), body weight and each measure of HRV for salt-insensitive and salt-sensitive women at baseline, and on low and high salt diets.
| Salt sensitive (N = 13) | Salt insensitive (N = 23) | |||||
|---|---|---|---|---|---|---|
| Baseline | Low Salt | High Salt | Baseline | Low Salt | High Salt | |
| SBP (mmHg) | 123.9 ± 8.9 | 116.0 ± 8.5 | 126.4 ±12.4 | 113.8 ± 9.2 | 114.5 ± 10.2 | 114.4 ± 10.7 |
| DBP (mmHg) | 73.5 ±7.7 | 71.0± 7.4 | 75.1 ±7.6 | 71.0 ± 6.0 | 72.6 ± 6.3 | 70.8 ± 5.7 |
| Br/M | 11.9 ± 3.6 | 12.1 ± 3.1 | 13.0 ± 2.4 | 14.9± 2.6 | 14.8 ± 2.5 | 15.5 ± 2.6 |
| TV (ml) | 337 ± 107 | 346 ± 145 | 395 ± 171 | 332 ± 152 | 318 ± 135 | 314 ± 148 |
| MV | 3.83 ± 1.17 | 4.54 ± 2.24 | 5.04 ± 2.10 | 5.80 ± 3.81 | 5.20 ± 3.19 | 5.47 ± 3.32 |
| Body Weight (Kg) | 69.3 ± 8.6 | 68.7 ± 8.4 | 69.4 ± 9.2 | 66.3 ± 8.7 | 65.6 ± 8.6 | 66.3 ± 8.7 |
| HR (bpm) | 64.7 ± 5.3 | 66.9 ± 8.4 | 64.0 ± 5.4 | 69.4 ± 8.0 | 72.9 ± 7.3 | 68.7 9.4 |
| LF (ln msec2) | 6.46 ± 0.81 | 5.94 ± 0.84 | 6.50 ± 0.72 | 6.39 ± 0.64 | 6.25 ± 0.66 | 6.39 ± 0.69 |
| HF (ln msec2) | 5.48 ± 0.40 | 4.83 ± 0.88 | 5.58 ± 0.69 | 5.89 ± 0.68 | 5.58 ± 0.64 | 6.01 ± 0.79 |
| LF/HF | 2.67 ± 1.44 | 3.61 ± 1.79 | 2.55 ± 1.20 | 1.85 ± 1.12 | 2.41 ± 1.31 | 1.57 ± 0.65 |
SS subjects were significantly older (58 ± 8 yrs) than SI (49 ± 7 yrs; t = 3.66; p < 0.001) subjects, but no significant differences between groups in body weight or BMI were observed.
Adherence: urinary volume and electrolyte excretion
Table 3 shows that mean 24-hr urinary volume was significantly greater on the high salt diet for the larger SI subgroup (t = 3.45; p < 0.001), though the increase was not significant for the smaller subgroup of SS subjects.
Table 3.
Means and standard deviations of 24 hr urine volume, sodium and potassium on low and high salt diet for salt-sensitive and salt insensitive-women on low and high salt.
| Salt-Sensitive (N = 13) | Salt-insensitive (N = 23) | |||
|---|---|---|---|---|
| Low Salt | High Salt | Low Salt | High Salt | |
| Urine Volume (ml) | 2300 ± 944 | 2525 ± 563 | 1838 ± 669 | 2543 ± 718* |
| Na + (mmol/24 hr) | 53 ± 32 | 220 ± 38* | 31 ± 12 | 238 ± 43* |
| K + (mmol/24 hr) | 71 ± 49 | 59 ± 19 | 65 ± 20 | 54 ± 13 |
Both SS (t = 12.12; p < 0.0001) and SI (t = 22.24; p < 0.0001) subjects showed a significant increase in sodium excretion on the high salt diet. Potassium excretion was not significantly different on high salt diet than on low salt diet for SS or SI subgroups.
Salt effects on group blood pressure and heart rate
Figure 1 provides a histogram of individual changes in systolic blood pressure from low to high salt diet. The range was from −6 to +21 mmHg, with 25 of the 36 subjects showing increases.
Figure 1.
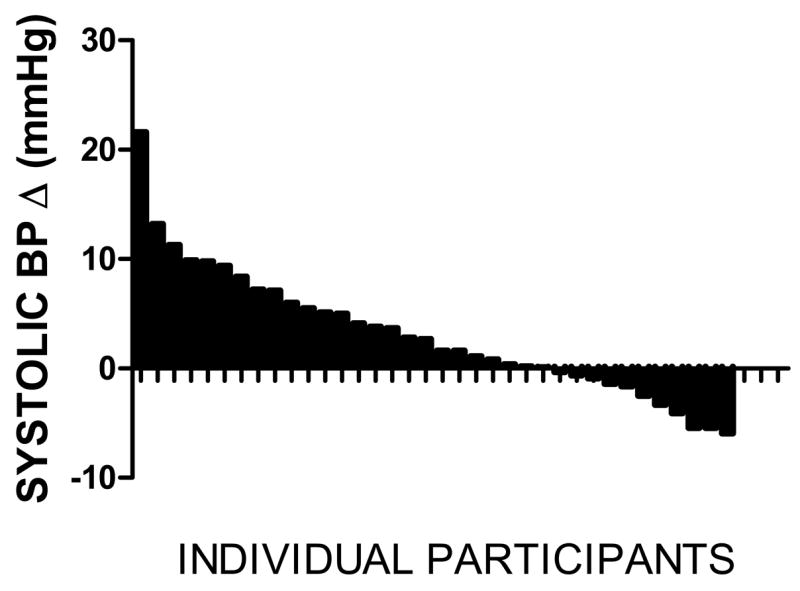
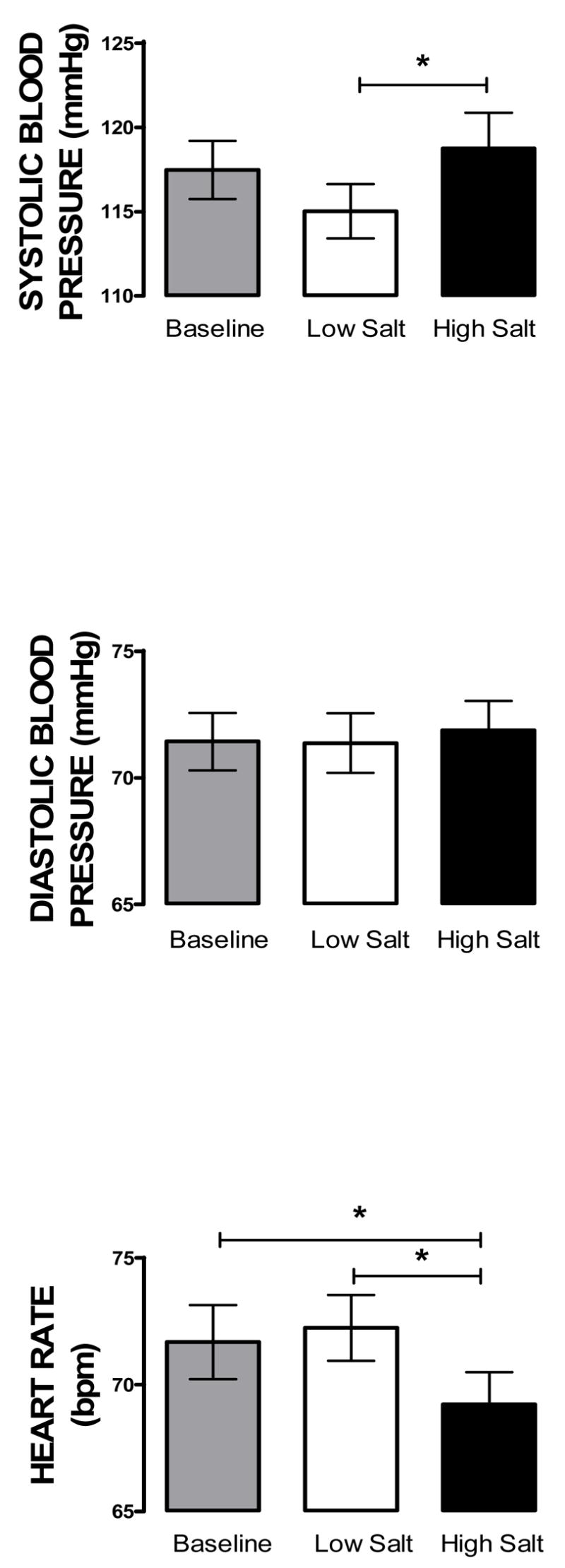
Figure 2 shows that mean systolic blood pressure (SBP) but not diastolic blood pressure (DBP), was significantly higher on high than low salt diet (F 2,35= 6.14; p < 0.005). Figure 2 also shows that mean heart rate on the high salt diet was significantly lower than at baseline or on low salt diet (F2,35 = 7.01 p < 0.005). The mean heart rates at baseline and on low salt diet were not significantly different from each other, however.
Figure 2.
Salt effects on heart rate variability
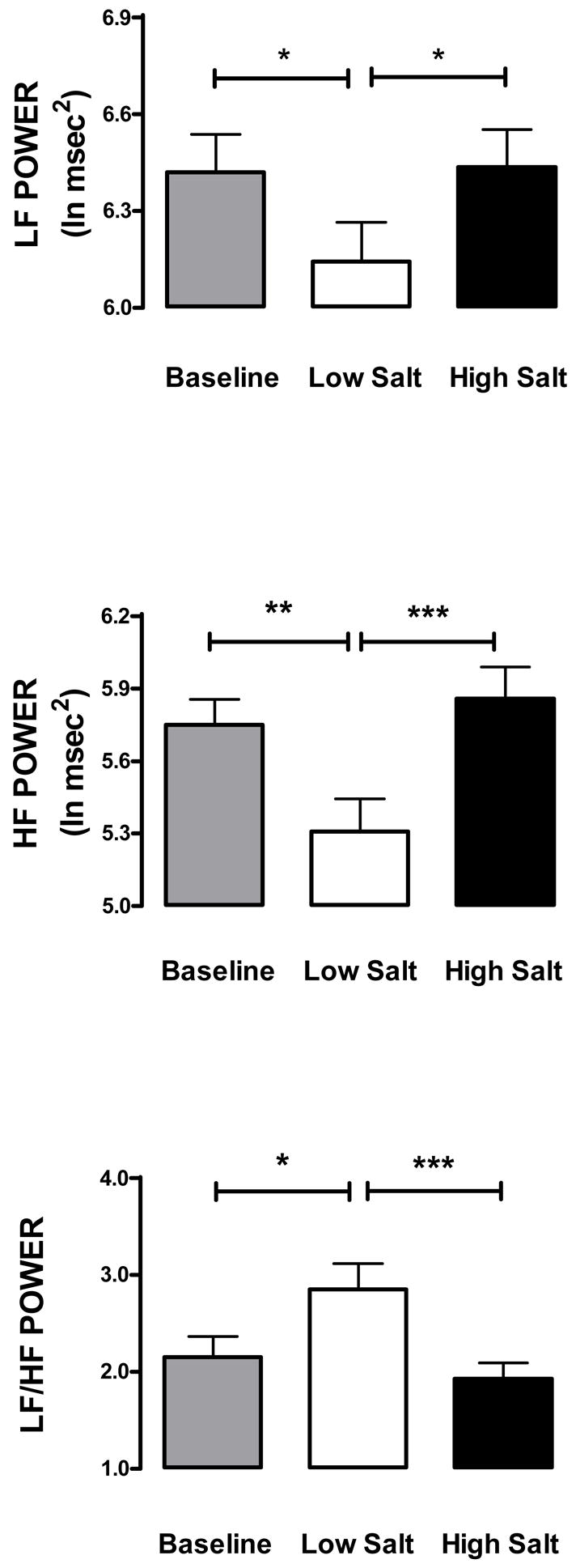
Table 2 and Figure 3 show that LF (F2,35 = 7.07; p < 0.002) and HF (F2,35 = 12.74; p < 0.001) were lower on low salt diet than at baseline or on high salt diet. LF/HF (F2,35 = 7.98; p < 0.001) was higher on low salt diet than at baseline or on high salt diet. No significant differences in LF, HF or LF/HF between baseline and high salt diet were observed.
Figure 3.
Association of HRV with blood pressure salt sensitivity
Figure 4 shows means and standard deviations of heart period (HP), a time domain estimate of respiratory sinus arrhythmia (RMSSD) and estimated resting cardiac vagal tone (using the equation developed by Grossman and Kollai 1993) for SS and SI subjects for the baseline condition. HP was greater in SS than SI subjects (t = 2.83; p < 0.005), RMSSD was lower in SS than SI subjects (t = 1.952 p < 0.05), and estimated cardiac vagal tone was greater in SS than in SI subjects (t = 2.71 p < 0.01).
DISCUSSION
The primary finding of this study was that high salt intake increased HF-HRV without changing respiratory rate or tidal volume, suggesting an increase in cardiac vagal tone on high salt diet. A second finding was that the SS subjects had greater cardiac vagal tone at rest than the SI group, as estimated from heart period and heart rate differences between groups (Grossman & Kollai, 1993).
Consistent with the modest salt-induced changes in heart rate, HF was decreased on low salt and increased on high salt diet. Moreover, the LF/HF ratio was greater on low than high salt diet, consistent with an increase in cardiac sympathetic tone during low salt diet. The apparent withdrawal of parasympathetic (HF) and increase in relative sympathetic (LF/HF) tone on high salt diet could reflect compensatory mechanisms responding to decreases in intravascular volume and blood pressure. That the changes in HRV associated with the changes in dietary sodium intake were not a byproduct of adaptation to the study conditions was indicated by the finding that baseline levels (in which sodium ingestion was most likely closer to high than low salt levels) demonstrated a reversal of effects over time.
These findings indicate that the heart and peripheral vasculature adapt to changes in plasma volume accompanying changes in sodium intake to maintain blood pressure, increasing cardiac activity and vascular tone when salt intake is low, and decreasing them when it is high. It has long been known that high salt intake by normotensive persons decreases circulating levels of norepinephrine and plasma renin activity, hormones that support sympathetic nervous system activity (e.g. Luft, Rankin, Henry & Weinberger, 1982). The urine volume increase on high salt diet could reflect increased thirst and drinking, an effect that has also been observed previously in another study of human salt loading (Bleys et al., 2006).
The present findings are consistent with previous studies of mental stress in young normotensive men. HF was lower in SS than SI subjects during mental challenge, and SS were also found to have lower respiratory rates than those of SI subjects (Buchholz et al, 2003). The authors of that study noted that if the effects of stress on HRV were mediated by differences in respiratory behavior, the effects should have been in the opposite direction. In the present study, the lack of significant changes in respiratory rate or tidal volume across salt conditions suggests that the HRV effects were not an artifact of breathing, but reflected changes in cardiac autonomic activity. This finding must be interpreted cautiously, however, since respiratory parameters can confound relations between respiratory sinus arrhythmia and cardiac vagal tone (Grossman & Taylor, 2007).
HRV is not a reliable indicator of individual differences in cardiac vagal tone, but the relationship between HRV and HP can be used to estimate it (Grossman & Kollai, 1993). The conclusion that SS subjects had greater cardiac vagal tone at rest might indicate greater tonic baroreceptor activity in the SS group secondary to increased peripheral resistance and blood pressure in those subjects. However, because respiration rate of SS subjects was also lower than in SI subjects, it is also possible that other central factors were responsible for the greater vagal efferent activity. In this regard, it is especially interesting that the cardiovascular reactivity of SS subjects to mental stress were greater than in SI subjects (Deter et al, 2001).
Whether the blood pressure responses to high salt intake involved autonomic effects parallel to those observed on cardiac function remains to be clarified. Sympathetic influence on vascular tone is complexly regulated, so that inferences from effects on cardiac activity cannot be made to the peripheral circulation (Tsuru, Tanimitsu & Hirai, 2002). Recent research has shown that salt loading of normotensive African Americans decreases total peripheral resistance in those subjects who are not salt-sensitive, but fails to produce a compensatory vasodilation in salt-sensitive subjects (Schmidlin, Sebastian & Morris, 2007). Recent studies also indicate that high salt intake can stimulate endogenous natriuretic factors that have vasoconstrictor properties, and could play a critical role in the development of salt-sensitive forms of hypertension (Haddy, Vanhoutte & Feletou, 2006). Additional research will be needed to clarify the extent to which peripheral adaptations to high salt intake in SS subjects might be mediated by such endogenous compounds.
As in virtually all other studies of this kind, individual blood pressure response to changes in dietary sodium intake constituted a continuum rather than a bimodal distribution. Thus, the designation of any particular magnitude of change in blood pressure as constituting salt sensitivity is somewhat arbitrary. The majority of women in this study were peri- or postmenopausal, most showed an increase in blood pressure on the high salt diet, and the group effect was statistically significant. The low potassium diet may have augmented the effects of high sodium intake on blood pressure, in part by decreasing urinary sodium excretion (Haddy, Vanhoutte & Feletou, 2006). No significant salt-induced changes in diastolic pressure were observed for the group. Unlike blood pressure, group mean heart rate under baseline conditions was more similar to that on low salt than on high salt conditions. The decrease in heart rate under conditions of high salt intake and increased blood pressure could be due to a baroreceptor response.
It should be noted that high salt diet might have different effects on HRV in hypertensive patients due to the changes in resting autonomic activity characteristic of this disorder (Brooks et al 2005). One previous study with hypertensive patients showed that high salt intake increased the HF and decreased the LF/HF components of HRV only in SI subjects (Minami, Kawano, Ishimitsu & Takishita, 1997). Moreover, SS patients with mild hypertension showed an impaired baroreceptor reflex after high salt intake (Coruzzi, et al., 2005), indicating a deficit in cardiac parasympathetic control. Structural changes in the cardiovascular system associated with the hypertensive adaptation could contribute to quantitative or qualitative differences from the HRV responses to salt observed in healthy individuals.
Interpretation of the present findings should be qualified in terms of a number of factors. First, potassium intake was kept constant, but carbohydrate and calcium intake differed between salt conditions. A very low potassium diet (10–16mmol/day) can elevate blood pressure in individuals with normal sodium intake (Adrogue & Madias, 2007). By standardizing potassium intake, dietary effects on HRV not attributable to changes in sodium intake should have been minimized. Carbohydrate feeding can increase sympathetic nervous system activity (Johnson, Zhang & Kotchen, 1993). Therefore, the apparent decreases in cardiac sympathetic activity on high salt diet should not have been mediated by the concurrent increases in carbohydrate intake. The lower dietary calcium on high salt intake could have amplified the effects of sodium on blood pressure, but should not have been a determinant of the greater HRV on high salt diet. No other differences between salt intake conditions were observed in macronutrient or mineral intake.
Second, only Caucasian women over age 40 were studied. The extent to which the results would also be observed in younger persons, men, and other racial groups needs to be determined. However, HRV can vary by age, sex and race, so that a homogenous population would be advantageous in avoiding these complexities. Third, the observed effects are specific to the time lines used in this study, and additional research is needed to determine how long such effects would be sustained. Finally, the extent to which HRV is a valid guide to autonomic balance remains a matter of debate (Ritz & Dahme, 2006).
In summary, this study supports the conclusion that high salt intake is associated with increased parasympathetic influence on cardiac function in normotensive women. It also found that SS and SI subjects differed in autonomic control of the heart, with SS persons having greater cardiac vagal tone at rest. The findings illustrate that sodium balance and associated changes in plasma volume are determinants of HRV, and should be so recognized in future behavioral studies of HRV. The extent to which cardiac parasympathetic activity is stably increased in SS persons is a topic for further investigation of relevance to the origins of sodium-sensitive forms of hypertension.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH National Institute on Aging. The authors thank John Sollers for his advice regarding the measurement of heart rate variability.
References
- Adrogue HJ, Madias MD. Sodium and Potasium in the Pathogenesis of hypertension. The New England Journal of Medicine. 2007;356:1966–1978. doi: 10.1056/NEJMra064486. [DOI] [PubMed] [Google Scholar]
- Anderson DE, Dhokalia A, Parsons D, Bagrov AY. Sodium sensitivity in young adults with resting end-tidal CO2. Journal of Hypertension. 1998;16:1015–1022. doi: 10.1097/00004872-199816070-00016. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Bleys J, Miller ER, III, Ange BA, Appel LJ. Effects of Dietary Sodium Intake and the Dietary Approaches to Stop Hypertension Diet on Renal Excretion of Water: Results from the DASH-Sodium Trial. Circulation. 2006;113(8):e317. (Abstract) [Google Scholar]
- Brooks VL, Haywood JR, Johnson AK. Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin Exp Pharmacol Physiol. 2005;32(5–6):426–432. doi: 10.1111/j.1440-1681.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- Buchholz K, Schachinger H, Wagner M, Sharma AM, Deter HC. Reduced vagal activity in salt-sensitive subjects during mental challenge. American Journal of Hypertension. 2003;16:531–536. doi: 10.1016/s0895-7061(03)00905-1. [DOI] [PubMed] [Google Scholar]
- Collins SM, Karasek RA, Costas K. Job strain and autonomic indices of cardiovascular disease risk. American Journal of Ind Medicine. 2005;48:182–193. doi: 10.1002/ajim.20204. [DOI] [PubMed] [Google Scholar]
- Coruzzi P, Parati G, Brambilla L, Brambilla V, Gualzeri M, Novarini A, Castiglioni P, Di Rienzo M. Effects of salt sensitivity on neural cardiovascular regulation in essential hypertension. Hypertension. 2005;46:1321–1326. doi: 10.1161/01.HYP.0000189183.50301.5c. [DOI] [PubMed] [Google Scholar]
- Deter HC, Buchholz K, Schorr U, Mathiak K, Sharma AM. Salt-sensitivity and other predictors of stress-related cardiovascular reactivity in healthy young males. Clinical and Experimental Hypertension. 2001;23:213–225. doi: 10.1081/ceh-100102661. [DOI] [PubMed] [Google Scholar]
- Deter HC, Micus C, Wagner M, Sharma AM, Buchholz K. Salt sensitivity, anxiety, and irritability predict blood pressure increase over five years in healthy males. Clinical and Experimental Hypertension. 2006;28:17–27. doi: 10.1080/10641960500386627. [DOI] [PubMed] [Google Scholar]
- Evans JM, Ziegler MG, Patwardham AR. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. Journal of Applied Physiology. 2001;91:2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- Falkner B, Kushner H. Effect of sodium loading on cardiovascular response in young blacks and whites. Hypertension. 1990;15:36–43. doi: 10.1161/01.hyp.15.1.36. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Bogart LM, Vranceanu AM, Wait LC. Job characteristics, occupational status, and ambulatory cardiovascular activity in women. Annals of Behavioral Medicine. 2004;28:62–73. doi: 10.1207/s15324796abm2801_8. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Salomon K, Zhou F, Owens JF, Edmundowicz D, Kuller LH, Matthews KA. A greater reduction in high-frequency heart rate variability to a psychological stressor is associated with subclinical coronary and aortic calcification in postmenopausal women. Psychosomatic Medicine. 2005;67:553–560. doi: 10.1097/01.psy.0000170335.92770.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Kollai M. Respiratory sinus arrhythmia, cardiac vagal tone, and respiration: within- and between –individual relations. Psychophysiology. 1993;(5):486–495. doi: 10.1111/j.1469-8986.1993.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: Relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2006;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Guasti L, Simoni C, Mainardi L, Crespi C, Cimpanelli M, Klersy C, Gaudio G, Grandi AM, Cerutti S, Venco A. Global link between heart arte and blood pressure oscillations at rest and during mental arousal in nomotensive and hypertensive subjects. Auton Neurosci. 2005;120:80–87. doi: 10.1016/j.autneu.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. American Journal of Physiology: Regulatory, Integrative and Comp Physiology. 2006;290:R546–R552. doi: 10.1152/ajpregu.00491.2005. [DOI] [PubMed] [Google Scholar]
- Haythornthwaite JA, Pratley RE, Anderson DE. Behavioral stress potentiates the blood pressure effects of high sodium diet. Psychosomatic Medicine. 1992;54(2):231–239. doi: 10.1097/00006842-199203000-00008. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Zhang HY, Kotchen TA. Sucrose does not raise blood pressure in rats maintained on a low salt diet. Hypertension. 1993;21:779–785. doi: 10.1161/01.hyp.21.6.779. [DOI] [PubMed] [Google Scholar]
- Kay SM, Marple SL. Spectrum analysis: a modern perspective. Processes of IEEE. 1981;69:1380–1419. [Google Scholar]
- Lucini G, De Vede G, Parati G, Pagani M. Impact of chronic psychosocial stress on autonomic cardiovascular regulation in otherwise healthy subjects. Hypertension. 2005;46:1201–1206. doi: 10.1161/01.HYP.0000185147.32385.4b. [DOI] [PubMed] [Google Scholar]
- Luft FC, Rankin LI, Henry DP, Weinberger MH. Sodium and the effects of norepinephrine. In: Fregly MJ, Kare MR, editors. The Role of Salt in Cardiovascular Hypertension. New York: Academic Press; 1982. pp. 267–279. [Google Scholar]
- Madden K, Savard GK. Effects of mental state on hear rate and blood pressure variability in men and women. Clinical Physiology. 1995;15:557–569. doi: 10.1111/j.1475-097x.1995.tb00544.x. [DOI] [PubMed] [Google Scholar]
- Minami J, Kawano Y, Tshimitsu T, Takishita S. Blunted parasympathetic modulation in salt-sensitive patients with essential hypertension: evaluation by power-spectral analysis of heart rate variability. Journal of Hypertension. 1997;15:727–735. doi: 10.1097/00004872-199715070-00004. [DOI] [PubMed] [Google Scholar]
- Mortara A, La Rovere MT, Signorini MG, Pantaleo P, Pinna G, Martinelli L, Ceconi C, Cerutti S, Tavazzi L. Can power spectral analysis of heart rate variability identify a high risk subgroup of congestive heart failure patients with excessive sympathetic activation? A pilot study before and after heart transplantation. Br Heart J. 1994;71:422–430. doi: 10.1136/hrt.71.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW. Digital Signal Processing. Englewood Cliffs, NJ: Prentice-Hall, Inc; 1975. [Google Scholar]
- Piccirillo G, Fimognari FL, Muizzi MR, Bucca C, Cacciafesta M, Marigliano V. Age-dependent influence on heart rate variability in salt-sensitive hypertensive subjects. Journal of the American Geriatric Society. 1996;44:530538. doi: 10.1111/j.1532-5415.1996.tb01438.x. [DOI] [PubMed] [Google Scholar]
- Reims HM, Sevre K, Hoieggen A, Fossum E, Eide I, Kjeldsen SE. Blood viscosity: effects of mental and relations to autonomic nervous system function and insulin sensitivity. Blood Pressure. 2005;14:159–169. doi: 10.1080/08037050510034176. [DOI] [PubMed] [Google Scholar]
- Ritz T, Dahme B. Implementation and interpretation of respiratory sinus arrhythmia measures in psychosomatic medicine: practice against better evidence. Psychosomatic Medicine. 2006;68:617–617. doi: 10.1097/01.psy.0000228010.96408.ed. [DOI] [PubMed] [Google Scholar]
- Stein PK, Kleiger RE. Insights from the study of Heart Rate Variability. Annual Review of Medicine. 1999;50:249–261. doi: 10.1146/annurev.med.50.1.249. [DOI] [PubMed] [Google Scholar]
- Schmidlin O, Sebastian AF, Morris RC. What initiates the pressor effect of salt-sensitive humans? Observations in normotensive blacks. Hypertension. 2007;49(5):1032–1039. doi: 10.1161/HYPERTENSIONAHA.106.084640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Prewitt RL, Ratts TE, Josephs JA, Connor MA. Hemodynamic characteristics of sodium-sensitive human subjects. Hypertension. 1988;9:k 398–406. doi: 10.1161/01.hyp.9.4.398. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology The North American Society of Pacing Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Tsuru H, Tanimitsu N, Hirai T. Role if perivascular sympathetic nerves and regional differences in the features of sympathetic innervation of vascular system. Jpn J Pharmacol. 2002;88:9–13. doi: 10.1254/jjp.88.9. [DOI] [PubMed] [Google Scholar]
- Virtanen R, Jula A, Kuusela T, Helenius H, Voipio-Pulkki LM. Reduced heart rate variability in hypertension: associations with lifestyle factors and plasma renin activity. Journal of Human Hypertension. 2003;17:171–179. doi: 10.1038/sj.jhh.1001529. [DOI] [PubMed] [Google Scholar]
- Weinberger MH. Sodium and blood pressure 2003. Current Opinions in Cardiology. 2004;19:353–356. doi: 10.1097/01.hco.0000127136.50978.db. [DOI] [PubMed] [Google Scholar]
- Weinberger MH, Fineberg NS. Sodium and volume sensitivity of blood pressure:age and pressure change over time. Hypertension. 1991;18:67–71. doi: 10.1161/01.hyp.18.1.67. [DOI] [PubMed] [Google Scholar]


