Abstract
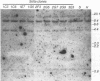
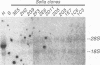
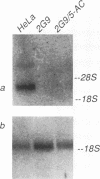
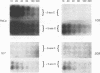
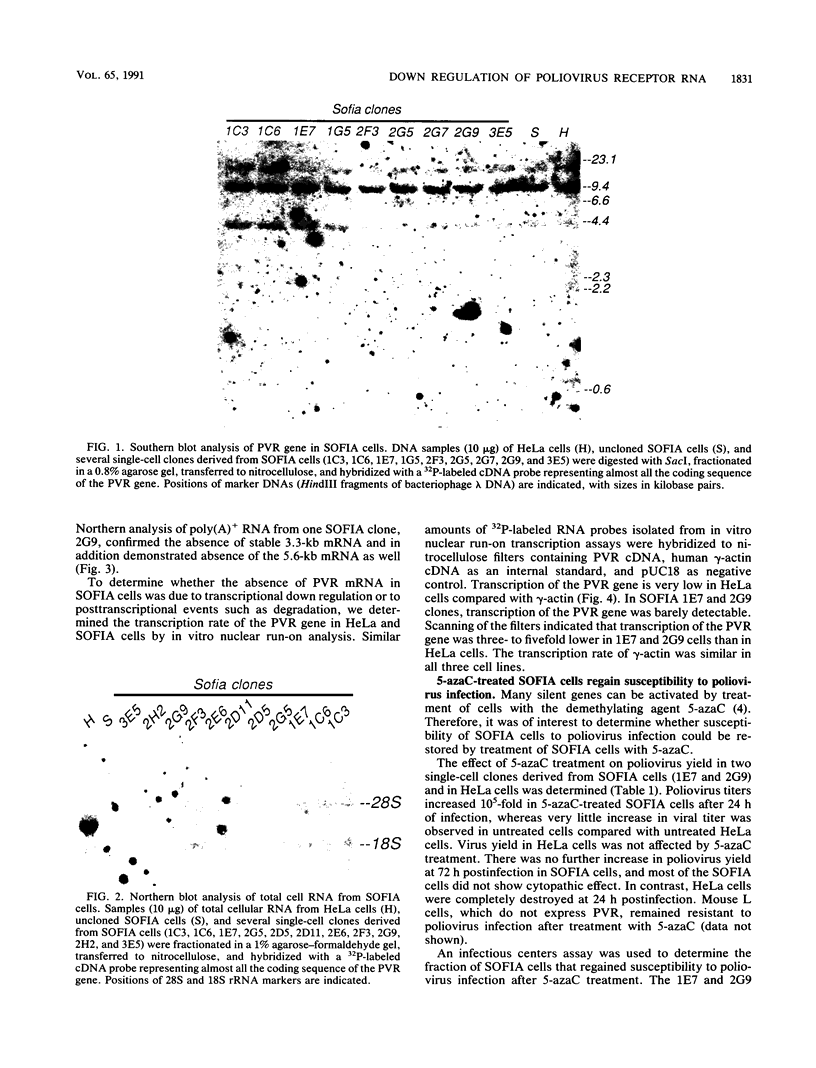
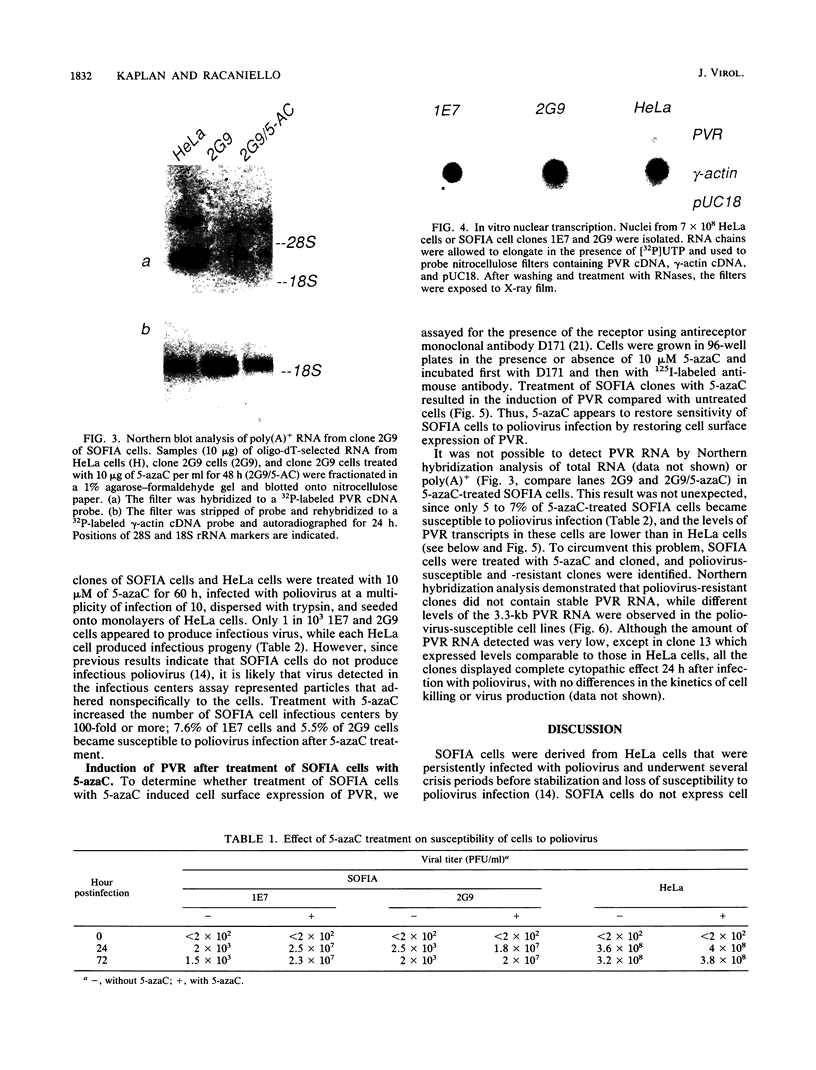
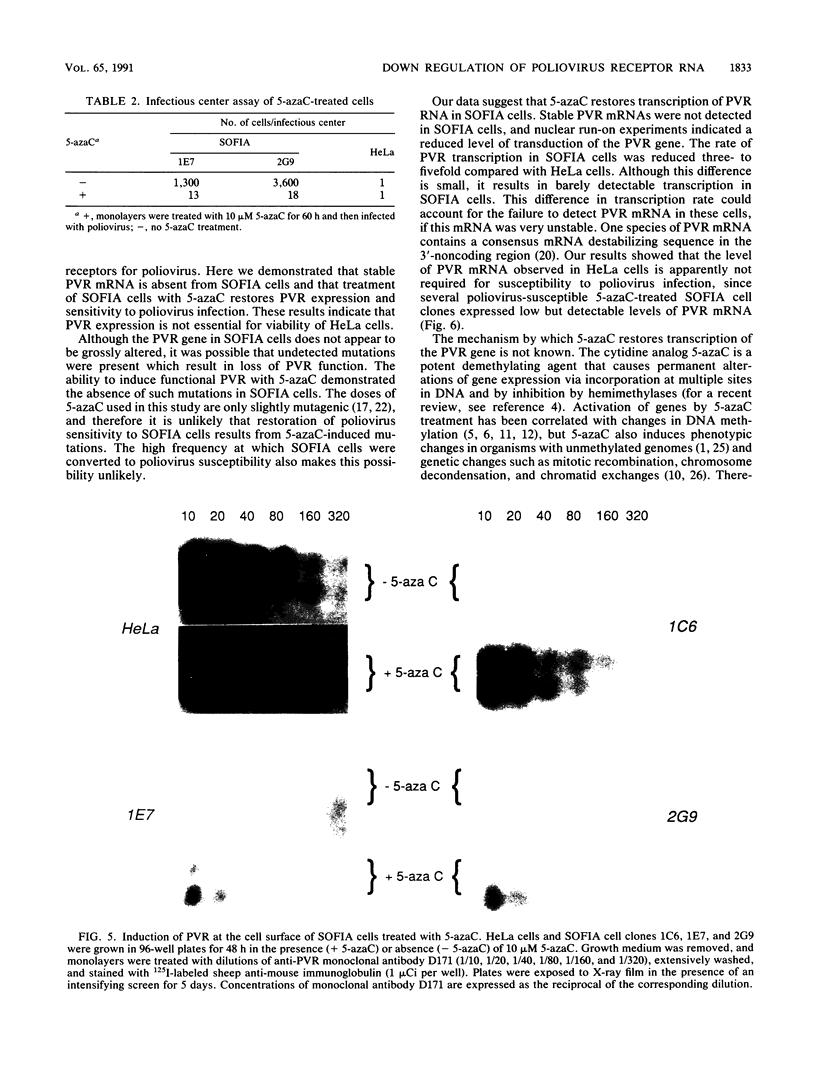
A line of HeLa cells (SOFIA) was previously isolated that is resistant to poliovirus infection and does not express functional virus binding sites at the cell surface. The expression of the poliovirus receptor (PVR) gene in SOFIA cells was examined to determine the molecular basis for the failure of these cells to express PVRs. Southern blot analysis of genomic DNA revealed that the PVR gene in SOFIA cells did not contain gross alterations. However, PVR transcripts were not detected in Northern (RNA) blot analysis of SOFIA cell RNA. In vitro nuclear run-on analysis showed that transcription of PVR-specific RNA was reduced in SOFIA cells. Treatment of SOFIA cells with 5-azacytidine restored susceptibility to poliovirus infection, which correlated with the appearance of PVRs at the cell surface, as detected with anti-PVR monoclonal antibody D171. PVR RNA was detected in clones derived from 5-azacytidine-treated SOFIA cells. SOFIA cells were converted to poliovirus sensitivity at a rate of 5 to 7%, suggesting that down regulation of PVR expression involved few cellular targets. Resistance of SOFIA cells to poliovirus infection therefore appears to result from down regulation of PVR RNA, leading to lack of PVR expression at the cell surface. Methylation may play a role in regulating the expression of the PVR gene, which is not essential for survival of HeLa cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antequera F., Tamame M., Villanueva J. R., Santos T. DNA methylation in the fungi. J Biol Chem. 1984 Jul 10;259(13):8033–8036. [PubMed] [Google Scholar]
- Cairo G., Bardella L., Schiaffonati L., Arosio P., Levi S., Bernelli-Zazzera A. Multiple mechanisms of iron-induced ferritin synthesis in HeLa cells. Biochem Biophys Res Commun. 1985 Nov 27;133(1):314–321. doi: 10.1016/0006-291x(85)91877-7. [DOI] [PubMed] [Google Scholar]
- Charache S., Dover G., Smith K., Talbot C. C., Jr, Moyer M., Boyer S. Treatment of sickle cell anemia with 5-azacytidine results in increased fetal hemoglobin production and is associated with nonrandom hypomethylation of DNA around the gamma-delta-beta-globin gene complex. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4842–4846. doi: 10.1073/pnas.80.15.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E., Karr R. W., Frost J. P., Gonwa T. A., Ginder G. D. Gamma interferon and 5-azacytidine cause transcriptional elevation of class I major histocompatibility complex gene expression in K562 leukemia cells in the absence of differentiation. Mol Cell Biol. 1986 May;6(5):1698–1705. doi: 10.1128/mcb.6.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compere S. J., McKnight G. S., Palmiter R. D. Androgens regulate ovomucoid and ovalbumin gene expression independently of estrogen. J Biol Chem. 1981 Jun 25;256(12):6341–6347. [PubMed] [Google Scholar]
- Constantinides P. G., Jones P. A., Gevers W. Functional striated muscle cells from non-myoblast precursors following 5-azacytidine treatment. Nature. 1977 May 26;267(5609):364–366. doi: 10.1038/267364a0. [DOI] [PubMed] [Google Scholar]
- Constantinides P. G., Taylor S. M., Jones P. A. Phenotypic conversion of cultured mouse embryo cells by aza pyrimidine nucleosides. Dev Biol. 1978 Sep;66(1):57–71. doi: 10.1016/0012-1606(78)90273-7. [DOI] [PubMed] [Google Scholar]
- Hori T. A. Induction of chromosome decondensation, sister-chromatid exchanges and endoreduplications by 5-azacytidine, an inhibitor of DNA methylation. Mutat Res. 1983 Jul;121(1):47–52. doi: 10.1016/0165-7992(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A., Holliday R. Azacytidine-induced reactivation of a DNA repair gene in Chinese hamster ovary cells. Mol Cell Biol. 1986 Aug;6(8):2944–2949. doi: 10.1128/mcb.6.8.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A. Altering gene expression with 5-azacytidine. Cell. 1985 Mar;40(3):485–486. doi: 10.1016/0092-8674(85)90192-8. [DOI] [PubMed] [Google Scholar]
- KAPLAN A. S. Comparison of susceptible and resistant cells to infection with poliomyelitis virus. Ann N Y Acad Sci. 1955 Sep 27;61(4):830-8; discussion, 838-9. doi: 10.1111/j.1749-6632.1955.tb42539.x. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Levy A., Racaniello V. R. Isolation and characterization of HeLa cell lines blocked at different steps in the poliovirus life cycle. J Virol. 1989 Jan;63(1):43–51. doi: 10.1128/jvi.63.1.43-51.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Racaniello V. R. Construction and characterization of poliovirus subgenomic replicons. J Virol. 1988 May;62(5):1687–1696. doi: 10.1128/jvi.62.5.1687-1696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Monica N., Meriam C., Racaniello V. R. Mapping of sequences required for mouse neurovirulence of poliovirus type 2 Lansing. J Virol. 1986 Feb;57(2):515–525. doi: 10.1128/jvi.57.2.515-525.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landolph J. R., Jones P. A. Mutagenicity of 5-azacytidine and related nucleosides in C3H/10T 1/2 clone 8 and V79 cells. Cancer Res. 1982 Mar;42(3):817–823. [PubMed] [Google Scholar]
- Mattia E., den Blaauwen J., Ashwell G., van Renswoude J. Multiple post-transcriptional regulatory mechanisms in ferritin gene expression. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1801–1805. doi: 10.1073/pnas.86.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn C. L., Wimmer E., Racaniello V. R. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Nobis P., Zibirre R., Meyer G., Kühne J., Warnecke G., Koch G. Production of a monoclonal antibody against an epitope on HeLa cells that is the functional poliovirus binding site. J Gen Virol. 1985 Dec;66(Pt 12):2563–2569. doi: 10.1099/0022-1317-66-12-2563. [DOI] [PubMed] [Google Scholar]
- Racaniello V. R., Meriam C. Poliovirus temperature-sensitive mutant containing a single nucleotide deletion in the 5'-noncoding region of the viral RNA. Virology. 1986 Dec;155(2):498–507. doi: 10.1016/0042-6822(86)90211-4. [DOI] [PubMed] [Google Scholar]
- Rao K., Harford J. B., Rouault T., McClelland A., Ruddle F. H., Klausner R. D. Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol. 1986 Jan;6(1):236–240. doi: 10.1128/mcb.6.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamame M., Antequera F., Santos E. Developmental characterization and chromosomal mapping of the 5-azacytidine-sensitive fluF locus of Aspergillus nidulans. Mol Cell Biol. 1988 Aug;8(8):3043–3050. doi: 10.1128/mcb.8.8.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann F. K., Scheel I. Genetic effects of 5-azacytidine in Saccharomyces cerevisiae. Mutat Res. 1984 Jan;139(1):21–24. doi: 10.1016/0165-7992(84)90116-7. [DOI] [PubMed] [Google Scholar]