Abstract
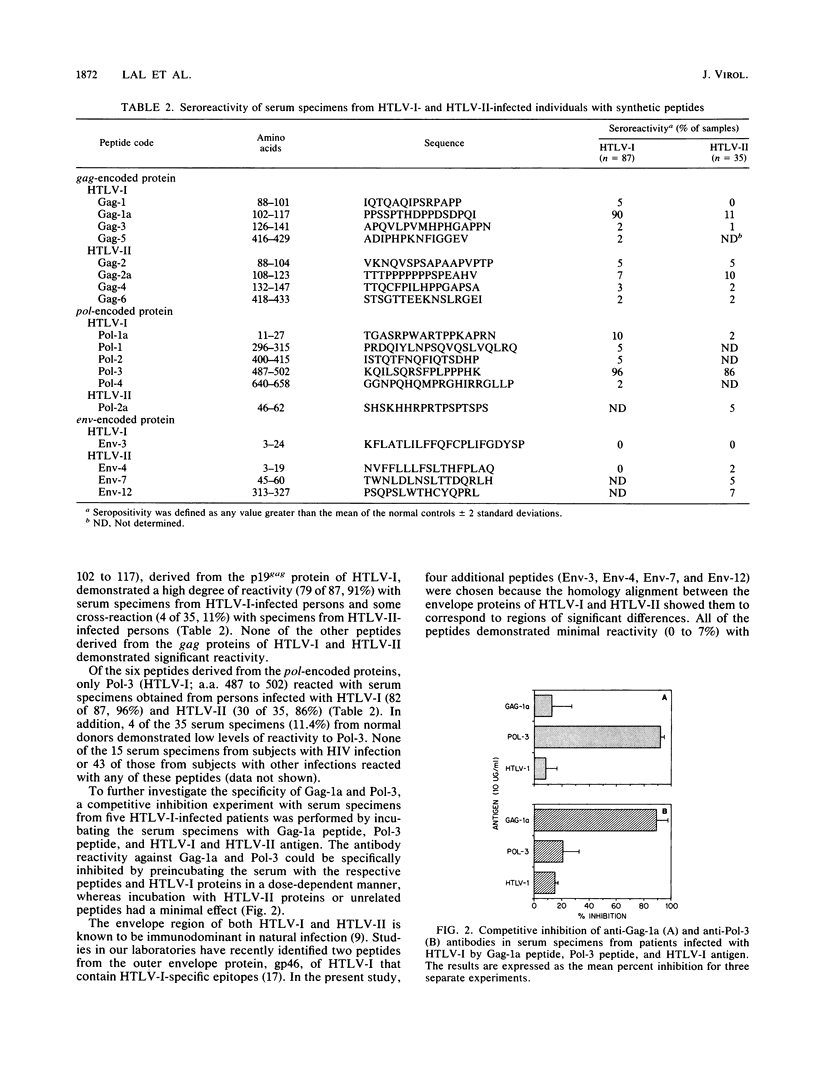
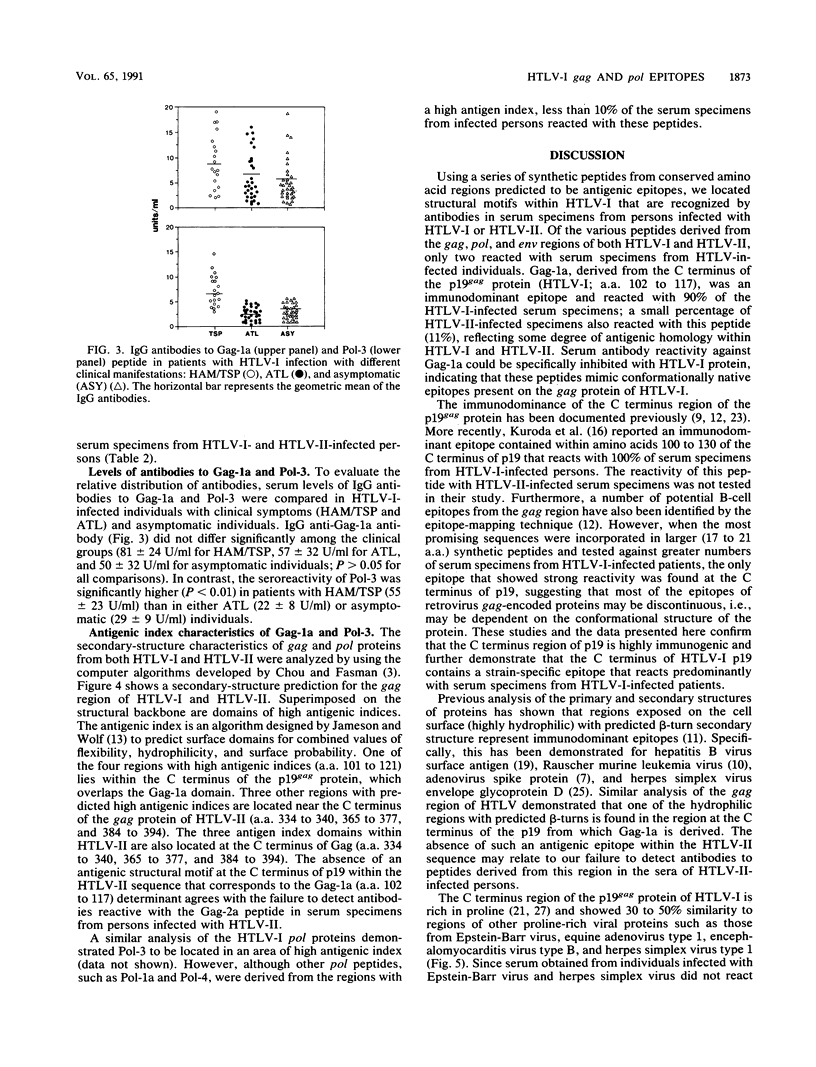
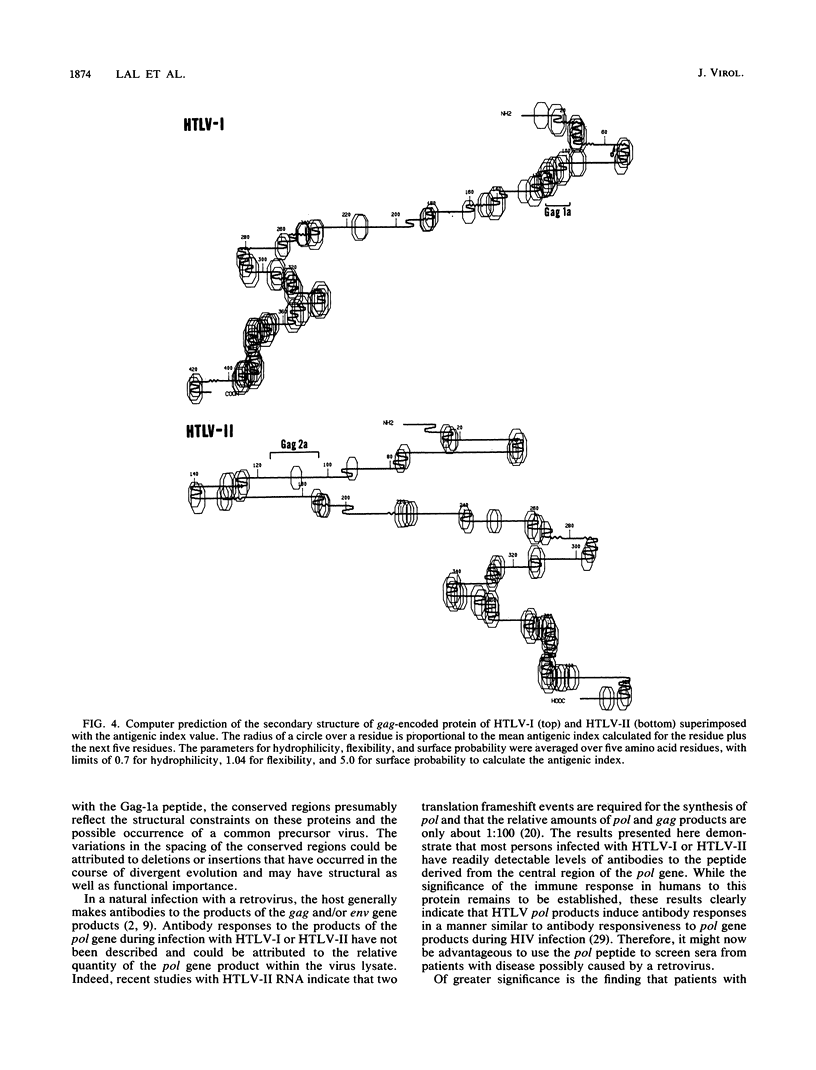
A series of synthetic peptides derived from the corresponding regions of the gag, pol, and env proteins of human T-cell lymphotropic virus types I (HTLV-I) and II (HTLV-II) were used in an enzyme immunoassay to map the immunodominant epitopes of HTLV. Serum specimens from 79 of 87 (91%) HTLV-I-infected patients reacted with the synthetic peptide Gag-1a (amino acids [a.a.] 102 to 117) derived from the C terminus of the p19gag protein of HTLV-I. Minimal cross-reactivity (11%) was observed with serum specimens from HTLV-II-infected patients. Peptide Pol-3, encoded by the pol region of HTLV-I (a.a. 487 to 502), reacted with serum specimens from both HTLV-I- and HTLV-II-infected patients (94 and 86%, respectively). The antibody levels to Pol-3 were significantly higher (P less than 0.01) in patients with HTLV-I-associated myelopathy/tropical spastic paraparesis than in either adult T-cell leukemia patients or HTLV-I-positive asymptomatic carriers. None of the other peptides studied demonstrated significant binding to serum specimens obtained from HTLV-I- or HTLV-II-infected individuals. While Gag-1a did not react with serum specimens from normal controls, Pol-3 demonstrated some reaction with specimens from seronegative individuals (11.4%). The antibodies to Gag-1a and Pol-3 in serum specimens from HTLV-I-infected patients could be specifically inhibited by the corresponding synthetic peptides and by a crude HTLV-I antigen preparation, indicating that these peptides mimic native epitopes present in HTLV-I proteins that are recognized by serum antibodies from HTLV-I- and -II-infected individuals.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. W., Epstein J. S., Lee T. H., Lairmore M. D., Saxinger C., Kalyanaraman V. S., Slamon D., Parks W., Poiesz B. J., Pierik L. T. Serological confirmation of human T-lymphotropic virus type I infection in healthy blood and plasma donors. Blood. 1989 Nov 15;74(7):2585–2591. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Varghese J. N., Laver W. G. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5;303(5912):41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- De B. K., Srinivasan A. Detection of human immunodeficiency virus (HIV) and human lymphotropic virus (HTLV) type I or II dual infections by polymerase chain reaction. Oncogene. 1989 Dec;4(12):1533–1535. [PubMed] [Google Scholar]
- Ehrlich G. D., Poiesz B. J. Clinical and molecular parameters of HTLV-I infection. Clin Lab Med. 1988 Mar;8(1):65–84. [PubMed] [Google Scholar]
- Gingeras T. R., Sciaky D., Gelinas R. E., Bing-Dong J., Yen C. E., Kelly M. M., Bullock P. A., Parsons B. L., O'Neill K. E., Roberts R. J. Nucleotide sequences from the adenovirus-2 genome. J Biol Chem. 1982 Nov 25;257(22):13475–13491. [PubMed] [Google Scholar]
- Hartley T. M., Khabbaz R. F., Cannon R. O., Kaplan J. E., Lairmore M. D. Characterization of antibody reactivity to human T-cell lymphotropic virus types I and II using immunoblot and radioimmunoprecipitation assays. J Clin Microbiol. 1990 Apr;28(4):646–650. doi: 10.1128/jcm.28.4.646-650.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseltine W. A., Sodroski J. G., Patarca R. Structure and function of the genome of HTLV. Curr Top Microbiol Immunol. 1985;115:177–209. doi: 10.1007/978-3-642-70113-9_12. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Sowder R. C., Smythers G. W., Oroszlan S. Primary structure of the low molecular weight nucleic acid-binding proteins of murine leukemia viruses. J Biol Chem. 1981 Aug 25;256(16):8400–8406. [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Robert-Guroff M., Miyoshi I., Golde D., Gallo R. C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science. 1982 Nov 5;218(4572):571–573. doi: 10.1126/science.6981847. [DOI] [PubMed] [Google Scholar]
- Khabbaz R. F., Darrow W. W., Hartley T. M., Witte J., Cohen J. B., French J., Gill P. S., Potterat J., Sikes R. K., Reich R. Seroprevalence and risk factors for HTLV-I/II infection among female prostitutes in the United States. JAMA. 1990 Jan 5;263(1):60–64. [PubMed] [Google Scholar]
- Kuroda N., Washitani Y., Shiraki H., Kiyokawa H., Ohno M., Sato H., Maeda Y. Detection of antibodies to human T-lymphotropic virus type I by using synthetic peptides. Int J Cancer. 1990 May 15;45(5):865–868. doi: 10.1002/ijc.2910450514. [DOI] [PubMed] [Google Scholar]
- Lal R. B., Rudolph D. L., Lairmore M. D., Khabbaz R. F., Garfield M., Coligan J. E., Folks T. M. Serologic discrimination of human T cell lymphotropic virus infection by using a synthetic peptide-based enzyme immunoassay. J Infect Dis. 1991 Jan;163(1):41–46. doi: 10.1093/infdis/163.1.41. [DOI] [PubMed] [Google Scholar]
- Lee H., Swanson P., Shorty V. S., Zack J. A., Rosenblatt J. D., Chen I. S. High rate of HTLV-II infection in seropositive i.v. drug abusers in New Orleans. Science. 1989 Apr 28;244(4903):471–475. doi: 10.1126/science.2655084. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Green N., Alexander H., Liu F. T., Sutcliffe J. G., Shinnick T. M. Chemically synthesized peptides predicted from the nucleotide sequence of the hepatitis B virus genome elicit antibodies reactive with the native envelope protein of Dane particles. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3403–3407. doi: 10.1073/pnas.78.6.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mador N., Panet A., Honigman A. Translation of gag, pro, and pol gene products of human T-cell leukemia virus type 2. J Virol. 1989 May;63(5):2400–2404. doi: 10.1128/jvi.63.5.2400-2404.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik K. T., Even J., Karpas A. Molecular cloning and complete nucleotide sequence of an adult T cell leukaemia virus/human T cell leukaemia virus type I (ATLV/HTLV-I) isolate of Caribbean origin: relationship to other members of the ATLV/HTLV-I subgroup. J Gen Virol. 1988 Jul;69(Pt 7):1695–1710. doi: 10.1099/0022-1317-69-7-1695. [DOI] [PubMed] [Google Scholar]
- Osame M., Matsumoto M., Usuku K., Izumo S., Ijichi N., Amitani H., Tara M., Igata A. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cell leukemialike cells. Ann Neurol. 1987 Feb;21(2):117–122. doi: 10.1002/ana.410210203. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Scearce R. M., Copeland T. D., Oroszlan S., Haynes B. F. C-terminal region of human T cell lymphotropic virus type I (HTLVI) p19 core protein is immunogenic in humans and contains an HTLVI-specific epitope. J Immunol. 1986 Apr 1;136(7):2393–2397. [PubMed] [Google Scholar]
- Pellett P. E., Kousoulas K. G., Pereira L., Roizman B. Anatomy of the herpes simplex virus 1 strain F glycoprotein B gene: primary sequence and predicted protein structure of the wild type and of monoclonal antibody-resistant mutants. J Virol. 1985 Jan;53(1):243–253. doi: 10.1128/jvi.53.1.243-253.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho H. M., Poiesz B., Ruscetti F. W., Gallo R. C. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology. 1981 Jul 15;112(1):355–360. doi: 10.1016/0042-6822(81)90642-5. [DOI] [PubMed] [Google Scholar]
- Schneider J., Yamamoto N., Hinuma Y., Hunsmann G. Sera from adult T-cell leukemia patients react with envelope and core polypeptides of adult T-cell leukemia virus. Virology. 1984 Jan 15;132(1):1–11. doi: 10.1016/0042-6822(84)90086-2. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimotohno K., Takahashi Y., Shimizu N., Gojobori T., Golde D. W., Chen I. S., Miwa M., Sugimura T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc Natl Acad Sci U S A. 1985 May;82(10):3101–3105. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer K. S., Higgins K. W., Powers M. A., Stephans J. C., Gyenes A., George-Nascimento C., Luciw P. A., Barr P. J., Hallewell R. A., Sanchez-Pescador R. Recombinant polypeptide from the endonuclease region of the acquired immune deficiency syndrome retrovirus polymerase (pol) gene detects serum antibodies in most infected individuals. J Virol. 1986 Apr;58(1):9–16. doi: 10.1128/jvi.58.1.9-16.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. E., Fang C. T., Slamon D. J., Poiesz B. J., Sandler S. G., Darr W. F., 2nd, Shulman G., McGowan E. I., Douglas D. K., Bowman R. J. Seroprevalence and epidemiological correlates of HTLV-I infection in U.S. blood donors. Science. 1988 Apr 29;240(4852):643–646. doi: 10.1126/science.2896386. [DOI] [PubMed] [Google Scholar]



