Abstract
The wild mouse ecotropic retrovirus CasBrE causes a spongiform neurodegenerative disease after neonatal inoculation, with an incubation period ranging from 2 to 12 months. We previously showed that introduction of long terminal repeat (LTR) and gag-pol sequences from a strain of Friend murine leukemia virus (FB29) resulted in a dramatic acceleration of the onset of the disease. The chimeric virus FrCasE, which consisted of the FB29 genome containing 3' pol and env sequences from the wild mouse virus, induced a highly predictable, lethal neurodegenerative disease with an incubation period of only 16 days. Here we report that the sequences which are primary determinants of the length of the incubation period are located in the 5' end of the viral genome between a KpnI site in the R region of the LTR and a PstI site immediately 5' of the start codon for pr65gag (R-U5-5' leader). This region contains the tRNA primer binding site, splice donor site for the subgenomic env mRNA, and the packaging sequence. Computer-assisted sequence analysis failed to find evidence of a consensus sequence for a DNA enhancer in this region. In addition, sequences within a region of the genome between a ClaI site at the 3' end of env to the KpnI site in the R region of the LTR (inclusive of U3) also influenced the incubation period of the disease, but the effect was distinctly weaker than that of the R-U5-5' leader sequence. This U3 effect, however, appeared to be independent of the number of direct repeats, since deletion of one of two duplicated 42-base repeats containing consensus sequences of nuclear-factor binding domains had no effect on the incubation period of the disease. On the basis of Southern blot analysis of total viral DNA in the tissues, the effect of these sequences on the incubation period appeared to be related to the level of virus replication in the central nervous system. All of the chimeric viruses analyzed, irrespective of neurovirulence, replicated to comparable levels in the spleen and induced comparable levels of viremia.
Full text
PDF


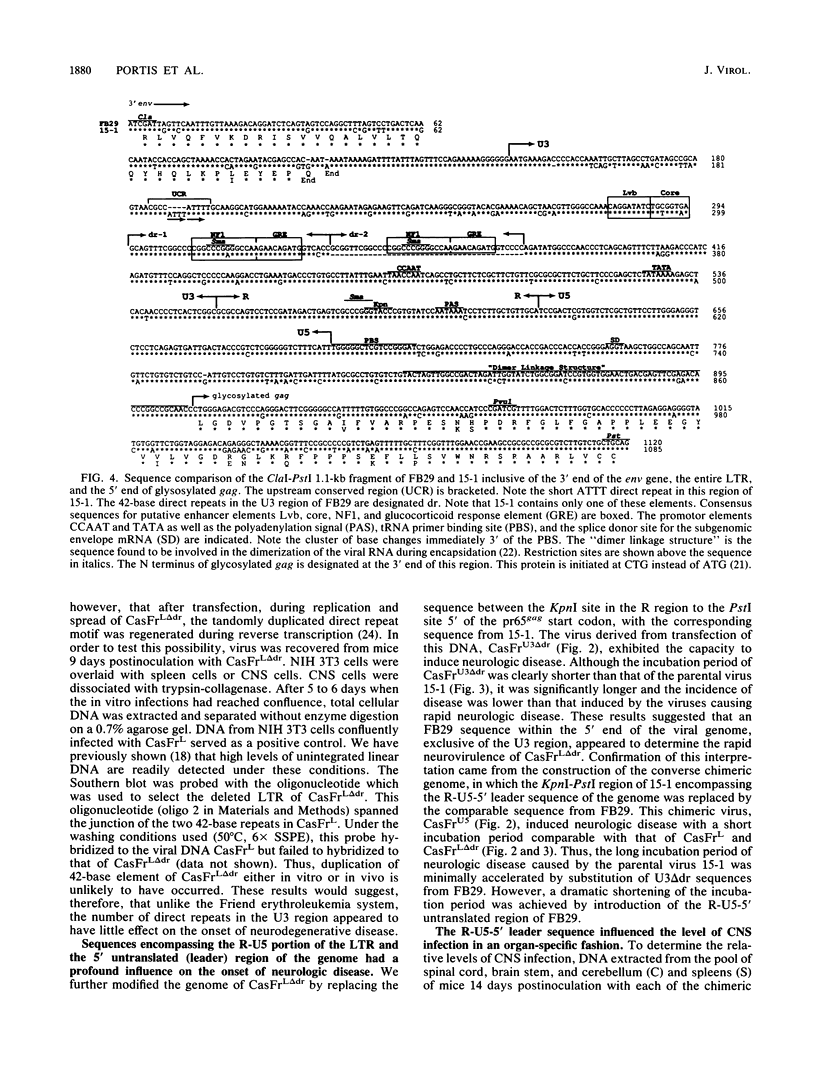



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews J. M., Gardner M. B. Lower motor neuron degeneration associated with type C RNA virus infection in mice: neuropathological features. J Neuropathol Exp Neurol. 1974 Apr;33(2):285–307. doi: 10.1097/00005072-197404000-00007. [DOI] [PubMed] [Google Scholar]
- Barklis E., Mulligan R. C., Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986 Nov 7;47(3):391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- Brooks B. R., Swarz J. R., Narayan O., Johnson R. T. Murine neurotropic retrovirus spongiform polioencephalomyelopathy: acceleration of disease by virus inoculum concentration. Infect Immun. 1979 Feb;23(2):540–544. doi: 10.1128/iai.23.2.540-544.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Blais B. P., Robinson H. L. Long terminal repeat (LTR) sequences, env, and a region near the 5' LTR influence the pathogenic potential of recombinants between Rous-associated virus types 0 and 1. J Virol. 1988 Sep;62(9):3431–3437. doi: 10.1128/jvi.62.9.3431-3437.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. W., Robinson H. L. Influence of avian leukosis viral sequences on transmission to the egg and embryo. Virology. 1989 Mar;169(1):222–226. doi: 10.1016/0042-6822(89)90059-7. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L., Barrette M., Jolicoeur P. Physical mapping of the paralysis-inducing determinant of a wild mouse ecotropic neurotropic retrovirus. J Virol. 1984 Nov;52(2):356–363. doi: 10.1128/jvi.52.2.356-363.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Chute H., Chao E., Feuerman M. Construction and characterization of Moloney murine leukemia virus mutants unable to synthesize glycosylated gag polyprotein. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5965–5969. doi: 10.1073/pnas.80.19.5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. R., Krieg A. M., Max E. E., Khan A. S. Negative control region at the 5' end of murine leukemia virus long terminal repeats. Mol Cell Biol. 1989 Feb;9(2):739–746. doi: 10.1128/mcb.9.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Chiri A., Dougherty M. F., Casagrande J., Estes J. D. Congenital transmission of murine leukemia virus from wild mice prone to the development of lymphoma and paralysis. J Natl Cancer Inst. 1979 Jan;62(1):63–70. [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Officer J. E., Rongey R. W., Parker J. C., Oliver C., Estes J. D., Huebner R. J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973 Oct;51(4):1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golemis E. A., Speck N. A., Hopkins N. Alignment of U3 region sequences of mammalian type C viruses: identification of highly conserved motifs and implications for enhancer design. J Virol. 1990 Feb;64(2):534–542. doi: 10.1128/jvi.64.2.534-542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golemis E., Li Y., Fredrickson T. N., Hartley J. W., Hopkins N. Distinct segments within the enhancer region collaborate to specify the type of leukemia induced by nondefective Friend and Moloney viruses. J Virol. 1989 Jan;63(1):328–337. doi: 10.1128/jvi.63.1.328-337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P. M., Ruscetti S. K., Morse H. C., 3rd Pathogenesis of paralysis and lymphoma associated with a wild mouse retrovirus infection. Part 1. Age- and dose-related effects in susceptible laboratory mice. J Neuroimmunol. 1981 Sep;1(3):275–285. doi: 10.1016/0165-5728(81)90031-x. [DOI] [PubMed] [Google Scholar]
- Li Y., Golemis E., Hartley J. W., Hopkins N. Disease specificity of nondefective Friend and Moloney murine leukemia viruses is controlled by a small number of nucleotides. J Virol. 1987 Mar;61(3):693–700. doi: 10.1128/jvi.61.3.693-700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette Y., Kay D. G., Rassart E., Robitaille Y., Jolicoeur P. Substitution of the U3 long terminal repeat region of the neurotropic Cas-Br-E retrovirus affects its disease-inducing potential. J Virol. 1990 Aug;64(8):3742–3752. doi: 10.1128/jvi.64.8.3742-3752.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L., Czub S., Garon C. F., McAtee F. J. Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J Virol. 1990 Apr;64(4):1648–1656. doi: 10.1128/jvi.64.4.1648-1656.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J., Hayes S. F. Horizontal transmission of murine retroviruses. J Virol. 1987 Apr;61(4):1037–1044. doi: 10.1128/jvi.61.4.1037-1044.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis J. L. Wild mouse retrovirus: pathogenesis. Curr Top Microbiol Immunol. 1990;160:11–27. doi: 10.1007/978-3-642-75267-4_2. [DOI] [PubMed] [Google Scholar]
- Prats A. C., De Billy G., Wang P., Darlix J. L. CUG initiation codon used for the synthesis of a cell surface antigen coded by the murine leukemia virus. J Mol Biol. 1989 Jan 20;205(2):363–372. doi: 10.1016/0022-2836(89)90347-1. [DOI] [PubMed] [Google Scholar]
- Prats A. C., Roy C., Wang P. A., Erard M., Housset V., Gabus C., Paoletti C., Darlix J. L. cis elements and trans-acting factors involved in dimer formation of murine leukemia virus RNA. J Virol. 1990 Feb;64(2):774–783. doi: 10.1128/jvi.64.2.774-783.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe A. H., Jaenisch R., Ruprecht R. M. Retroviruses and mouse embryos: a rapid model for neurovirulence and transplacental antiviral therapy. Science. 1987 Jun 26;236(4809):1671–1674. doi: 10.1126/science.3037694. [DOI] [PubMed] [Google Scholar]
- Spiro C., Li J. P., Bestwick R. K., Kabat D. An enhancer sequence instability that diversifies the cell repertoire for expression of a murine leukemia virus. Virology. 1988 Jun;164(2):350–361. doi: 10.1016/0042-6822(88)90548-x. [DOI] [PubMed] [Google Scholar]
- Teich N. M., Weiss R. A., Martin G. R., Lowy D. R. Virus infection of murine teratocarcinoma stem cell lines. Cell. 1977 Dec;12(4):973–982. doi: 10.1016/0092-8674(77)90162-3. [DOI] [PubMed] [Google Scholar]
- Weiher H., Barklis E., Ostertag W., Jaenisch R. Two distinct sequence elements mediate retroviral gene expression in embryonal carcinoma cells. J Virol. 1987 Sep;61(9):2742–2746. doi: 10.1128/jvi.61.9.2742-2746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]




