Abstract
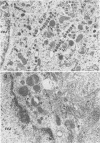
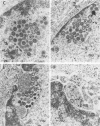
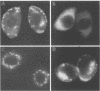
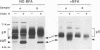
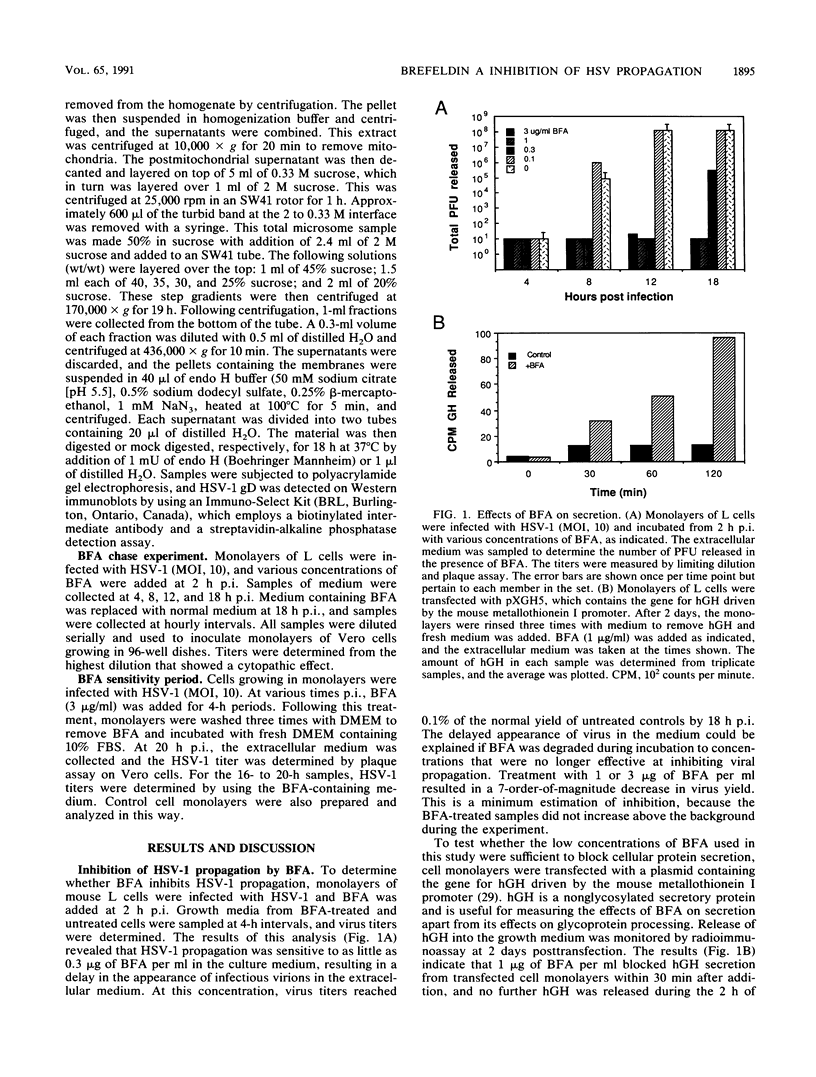
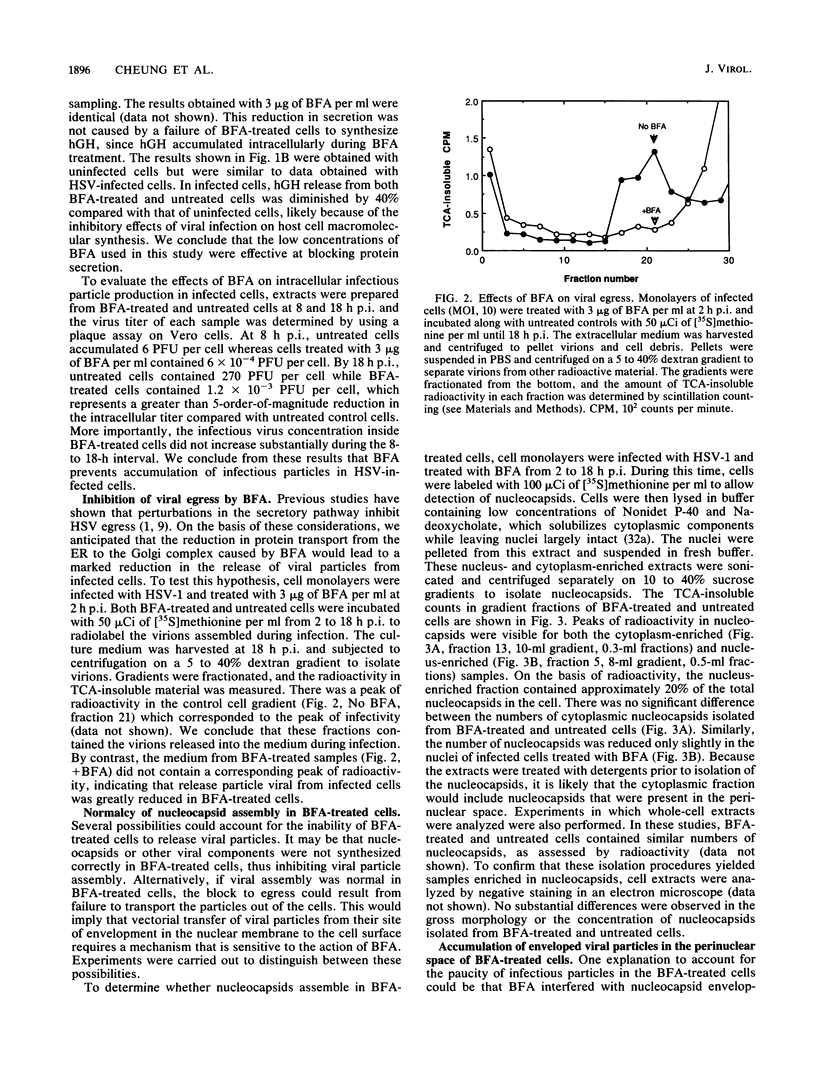
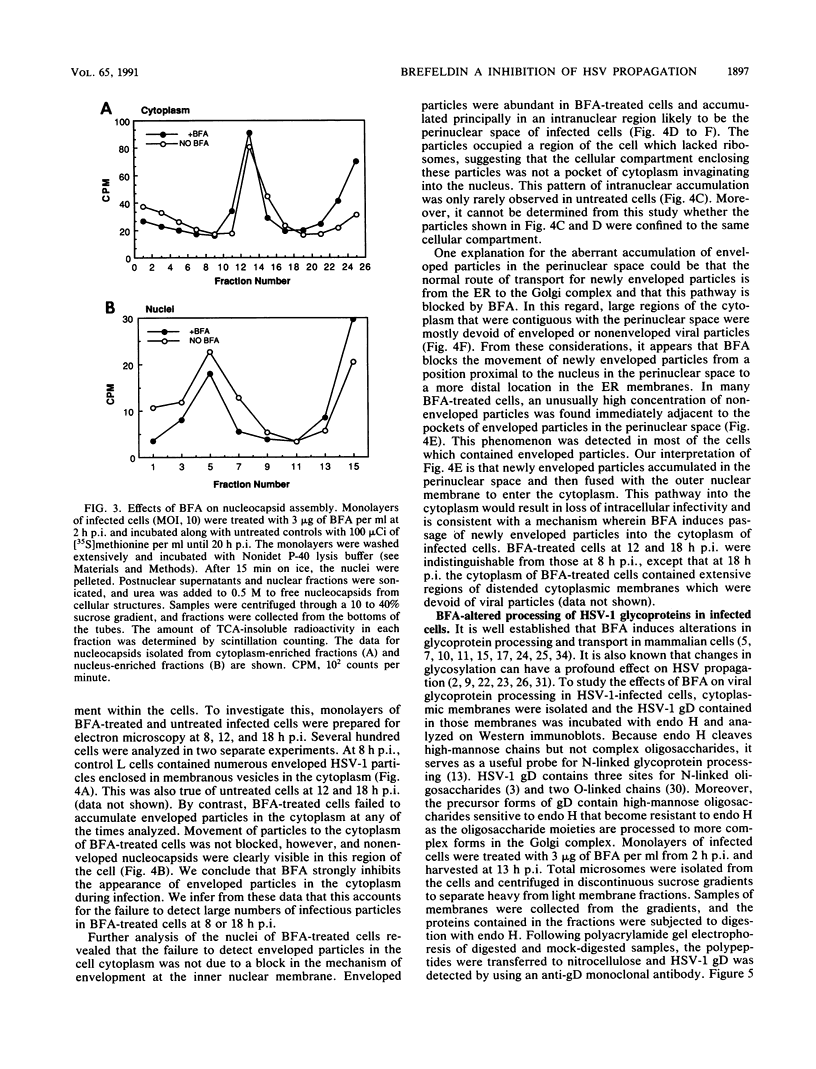
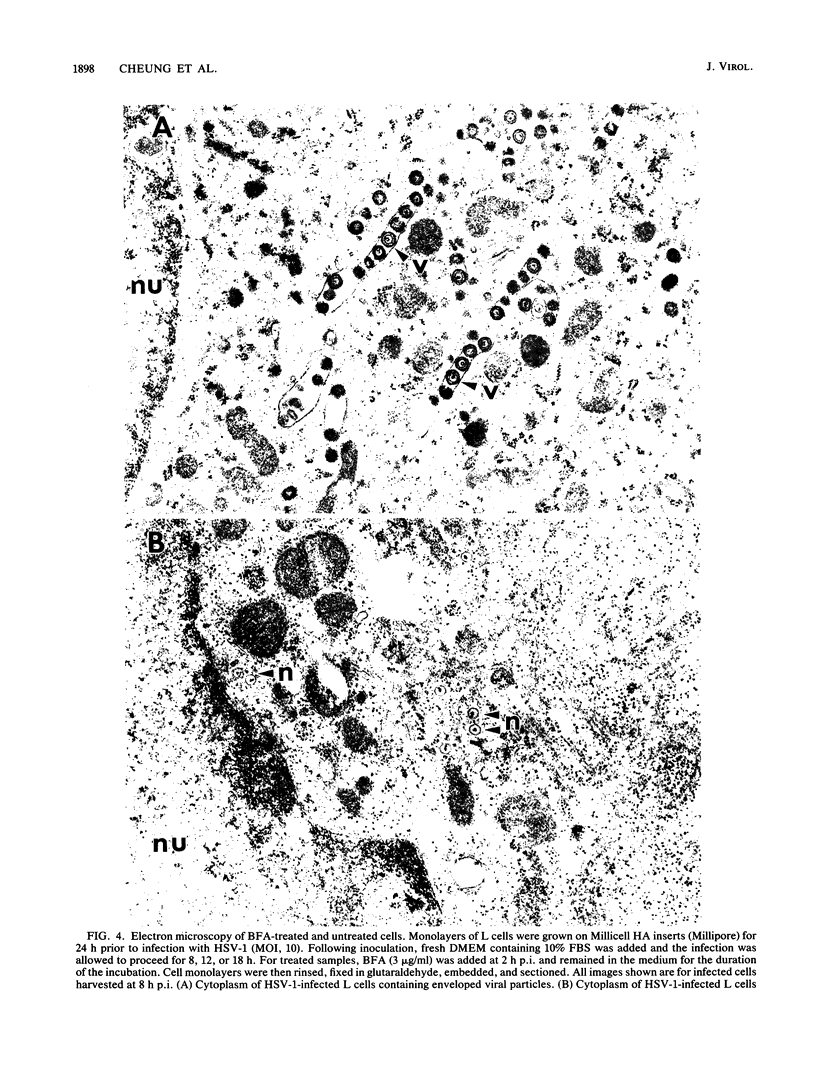
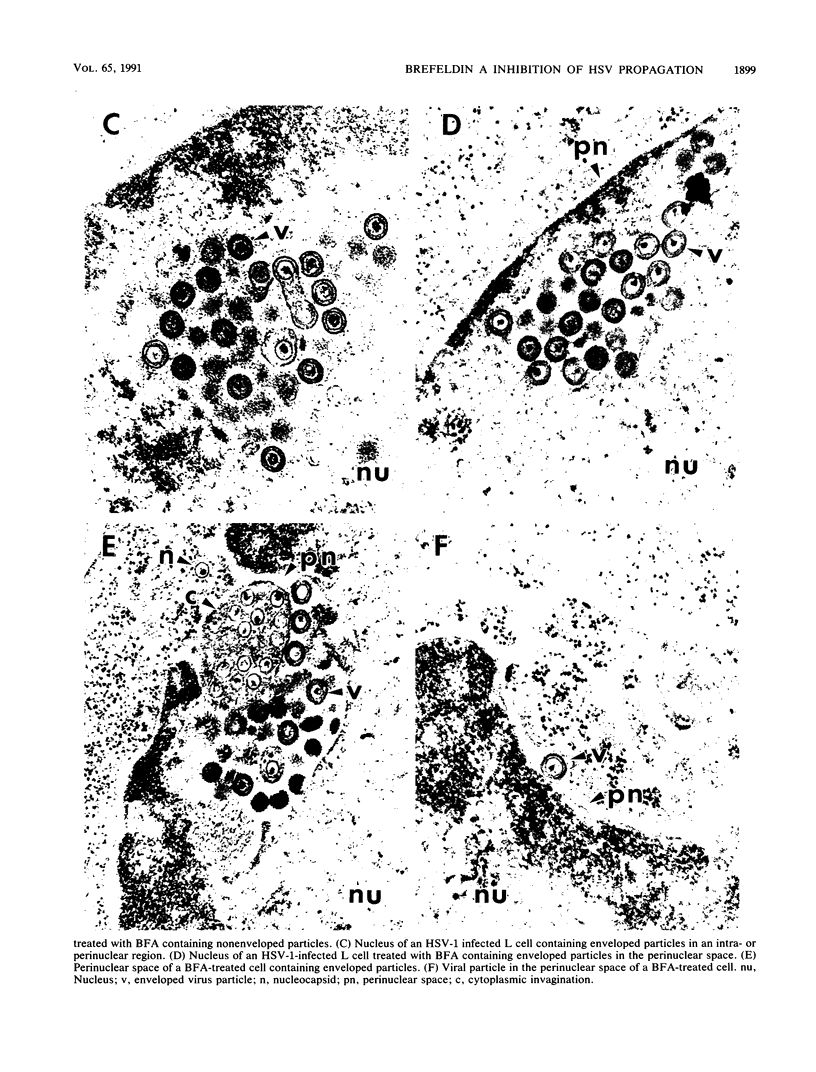
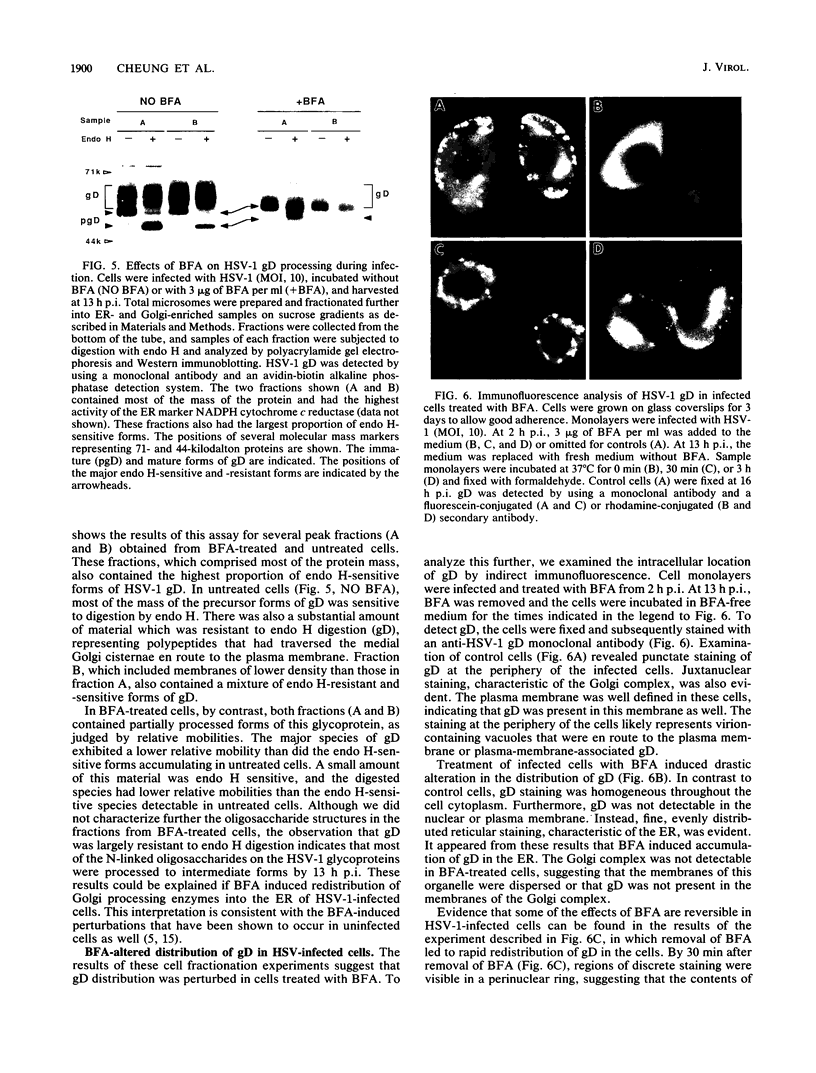
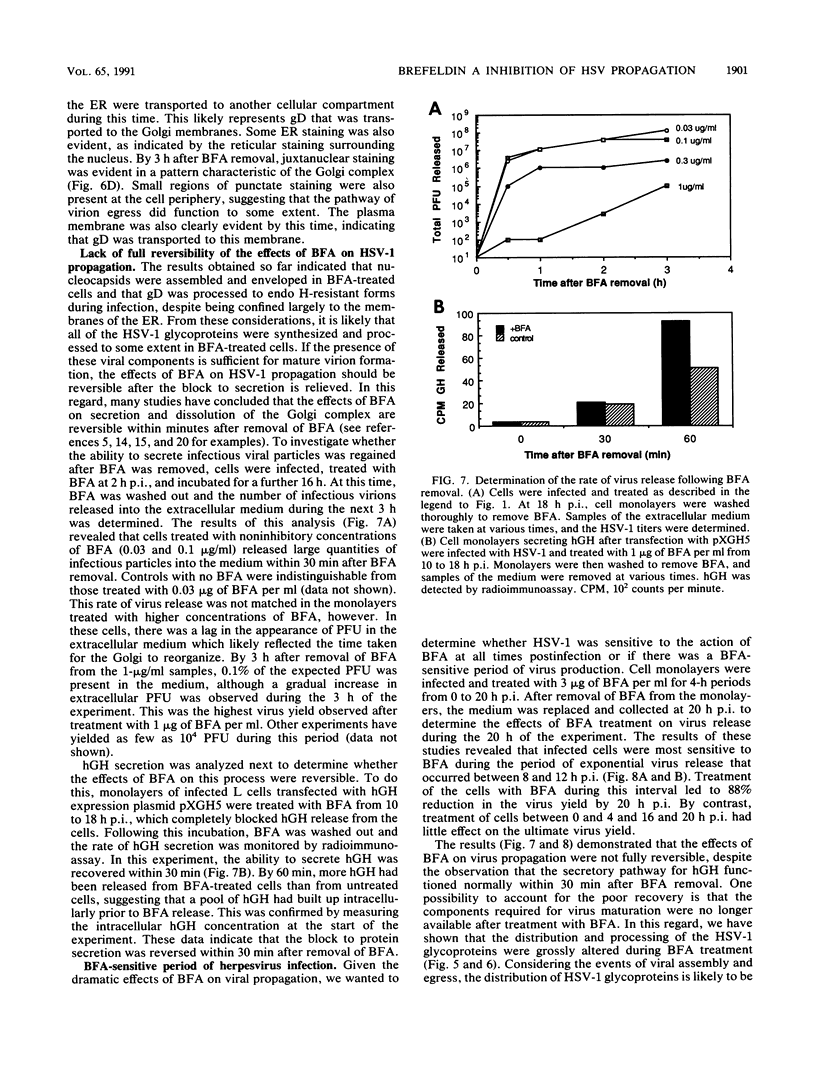
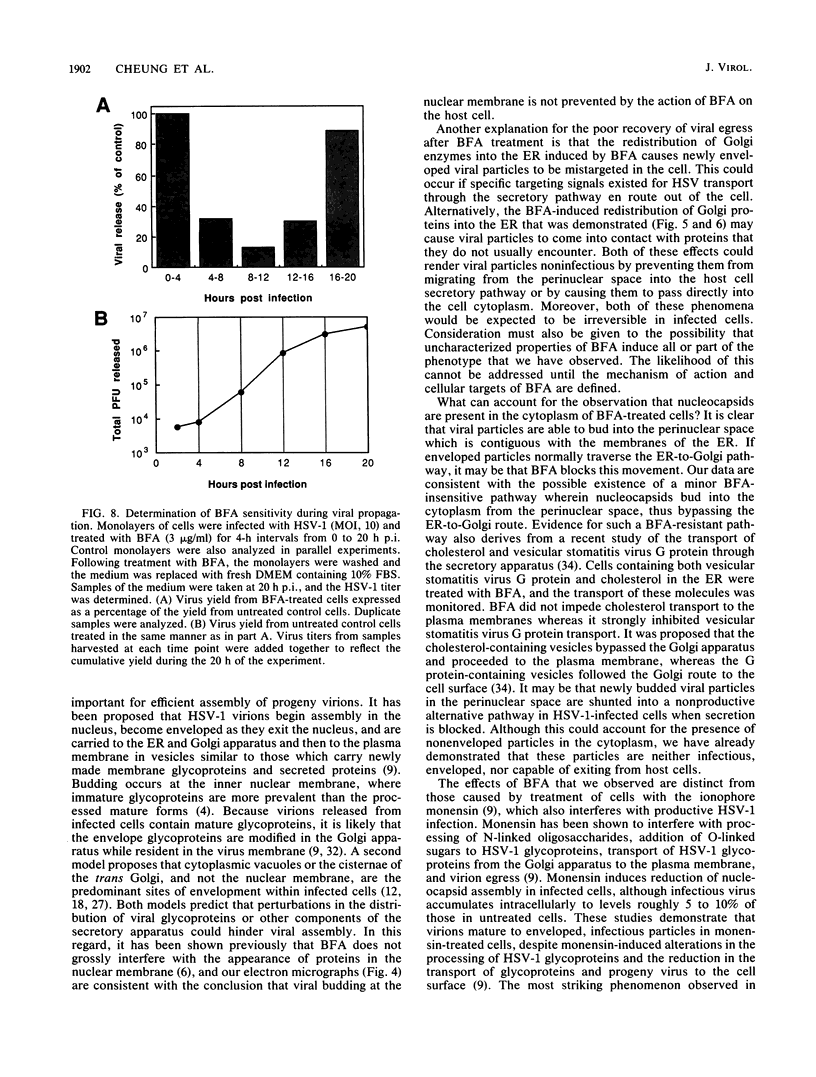
Herpes simplex virus (HSV) requires the host cell secretory apparatus for transport and processing of membrane glycoproteins during the course of virus assembly. Brefeldin A (BFA) has been reported to induce retrograde movement of molecules from the Golgi to the endoplasmic reticulum and to cause disassembly of the Golgi complex. We examined the effects of BFA on propagation of HSV type 1. Release of virions into the extracellular medium was blocked by as little as 0.3 microgram of BFA per ml when present from 2 h postinfection. Characterization of infected cells revealed that BFA inhibited infectious viral particle formation without affecting nucleocapsid formation. Electron microscopic analyses of BFA-treated and untreated cells (as in control cells) demonstrated that viral particles were enveloped at the inner nuclear membrane in BFA-treated cells and accumulated aberrantly in this region. Most of the progeny virus particles observed in the cytoplasm of control cells, but not that of BFA-treated cells, were enveloped and contained within membrane vesicles, whereas many unenveloped nucleocapsids were detected in the cytoplasm of BFA-treated cells. This suggests that BFA prevents the transport of enveloped particles from the perinuclear space to the cytoplasmic vesicles. These findings indicate that BFA-induced retrograde movement of molecules from the Golgi complex to the endoplasmic reticulum early in infection arrests the ability of host cells to support maturation and egress of enveloped viral particles. Furthermore, we demonstrate that the effects of BFA on HSV propagation are not fully reversible, indicating that maturation and egress of HSV type 1 particles relies on a series of events which cannot be easily reconstituted after the block to secretion is relieved.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banfield B. W., Tufaro F. Herpes simplex virus particles are unable to traverse the secretory pathway in the mouse L-cell mutant gro29. J Virol. 1990 Dec;64(12):5716–5729. doi: 10.1128/jvi.64.12.5716-5729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Poletti L., Dall'Olio F., Serafini-Cessi F. Infectivity and glycoprotein processing of herpes simplex virus type 1 grown in a ricin-resistant cell line deficient in N-acetylglucosaminyl transferase I. J Virol. 1982 Sep;43(3):1061–1071. doi: 10.1128/jvi.43.3.1061-1071.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Matthews J. T., May M., Eisenberg R. Glycopeptides of the type-common glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1983 Jun;46(3):679–689. doi: 10.1128/jvi.46.3.679-689.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T., Courtney R. J. Virus-specific glycoproteins associated with the nuclear fraction of herpes simplex virus type 1-infected cells. J Virol. 1984 Feb;49(2):594–597. doi: 10.1128/jvi.49.2.594-597.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms R. W., Russ G., Yewdell J. W. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989 Jul;109(1):61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara T., Oda K., Ikehara Y. Dynamic distribution of the Golgi marker thiamine pyrophosphatase is modulated by brefeldin A in rat hepatoma cells. Cell Struct Funct. 1989 Oct;14(5):605–616. doi: 10.1247/csf.14.605. [DOI] [PubMed] [Google Scholar]
- Gamou S., Shimizu N. Glycosylation of the epidermal growth factor receptor and its relationship to membrane transport and ligand binding. J Biochem. 1988 Sep;104(3):388–396. doi: 10.1093/oxfordjournals.jbchem.a122478. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Ito S., Noguchi T., Naito H. Effects of brefeldin A on the synthesis and secretion of egg white proteins in primary cultured oviduct cells of laying Japanese quail (Coturnix coturnix japonica). Biochim Biophys Acta. 1989 Apr 25;991(1):36–43. doi: 10.1016/0304-4165(89)90025-1. [DOI] [PubMed] [Google Scholar]
- Kato S., Noguchi T., Naito H. Secretion of egg white proteins in primary cultured oviduct cells of laying Japanese quail (Coturnix coturnix japonica). Poult Sci. 1987 Jul;66(7):1208–1216. doi: 10.3382/ps.0661208. [DOI] [PubMed] [Google Scholar]
- Komuro M., Tajima M., Kato K. Transformation of Golgi membrane into the envelope of herpes simplex virus in rat anterior pituitary cells. Eur J Cell Biol. 1989 Dec;50(2):398–406. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989 Mar 10;56(5):801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopata M. A., Cleveland D. W., Sollner-Webb B. High level transient expression of a chloramphenicol acetyl transferase gene by DEAE-dextran mediated DNA transfection coupled with a dimethyl sulfoxide or glycerol shock treatment. Nucleic Acids Res. 1984 Jul 25;12(14):5707–5717. doi: 10.1093/nar/12.14.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misumi Y., Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986 Aug 25;261(24):11398–11403. [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuchtern J. G., Bonifacino J. S., Biddison W. E., Klausner R. D. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature. 1989 May 18;339(6221):223–226. doi: 10.1038/339223a0. [DOI] [PubMed] [Google Scholar]
- Oda K., Fujiwara T., Ikehara Y. Brefeldin A arrests the intracellular transport of viral envelope proteins in primary cultured rat hepatocytes and HepG2 cells. Biochem J. 1990 Jan 1;265(1):161–167. doi: 10.1042/bj2650161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Hirose S., Takami N., Misumi Y., Takatsuki A., Ikehara Y. Brefeldin A arrests the intracellular transport of a precursor of complement C3 before its conversion site in rat hepatocytes. FEBS Lett. 1987 Apr 6;214(1):135–138. doi: 10.1016/0014-5793(87)80028-5. [DOI] [PubMed] [Google Scholar]
- Olofsson S., Milla M., Hirschberg C., De Clercq E., Datema R. Inhibition of terminal N- and O-glycosylation specific for herpesvirus-infected cells: mechanism of an inhibitor of sugar nucleotide transport across Golgi membranes. Virology. 1988 Oct;166(2):440–450. doi: 10.1016/0042-6822(88)90515-6. [DOI] [PubMed] [Google Scholar]
- Peake M. L., Nystrom P., Pizer L. I. Herpesvirus glycoprotein synthesis and insertion into plasma membranes. J Virol. 1982 May;42(2):678–690. doi: 10.1128/jvi.42.2.678-690.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel V. S., Liu A. Y., Miura Y., Magner J. A. The effects of brefeldin-A on the high mannose oligosaccharides of mouse thyrotropin, free alpha-subunits, and total glycoproteins. Endocrinology. 1988 Jul;123(1):310–318. doi: 10.1210/endo-123-1-310. [DOI] [PubMed] [Google Scholar]
- Perkel V. S., Miura Y., Magner J. A. Brefeldin A inhibits oligosaccharide processing of glycoproteins in mouse hypothyroid pituitary tissue at several subcellular sites. Proc Soc Exp Biol Med. 1989 Mar;190(3):286–293. doi: 10.3181/00379727-190-42862. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M., Dubois-Dalcq M. Intramembrane changes occurring during maturation of herpes simplex virus type 1: freeze-fracture study. J Virol. 1978 May;26(2):435–447. doi: 10.1128/jvi.26.2.435-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Palade G. E., Farquhar M. G. Antibodies to rat pancreas Golgi subfractions: identification of a 58-kD cis-Golgi protein. J Cell Biol. 1987 Nov;105(5):2021–2029. doi: 10.1083/jcb.105.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F., Malagolini N., Pereira L., Campadelli-Fiume G. Comparative study on O-linked oligosaccharides of glycoprotein D of herpes simplex virus types 1 and 2. J Gen Virol. 1988 Apr;69(Pt 4):869–877. doi: 10.1099/0022-1317-69-4-869. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F., Scannavini M., Campadelli-Fiume G. Processing of herpes simplex virus-1 glycans in cells defective in glycosyl transferases of the Golgi system: relationship to cell fusion and virion egress. Virology. 1983 Nov;131(1):59–70. doi: 10.1016/0042-6822(83)90533-0. [DOI] [PubMed] [Google Scholar]
- Tufaro F., Snider M. D., McKnight S. L. Identification and characterization of a mouse cell mutant defective in the intracellular transport of glycoproteins. J Cell Biol. 1987 Aug;105(2):647–657. doi: 10.1083/jcb.105.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani L., Simoni R. D. Cholesterol and vesicular stomatitis virus G protein take separate routes from the endoplasmic reticulum to the plasma membrane. J Biol Chem. 1990 Feb 5;265(4):1919–1923. [PubMed] [Google Scholar]