Abstract
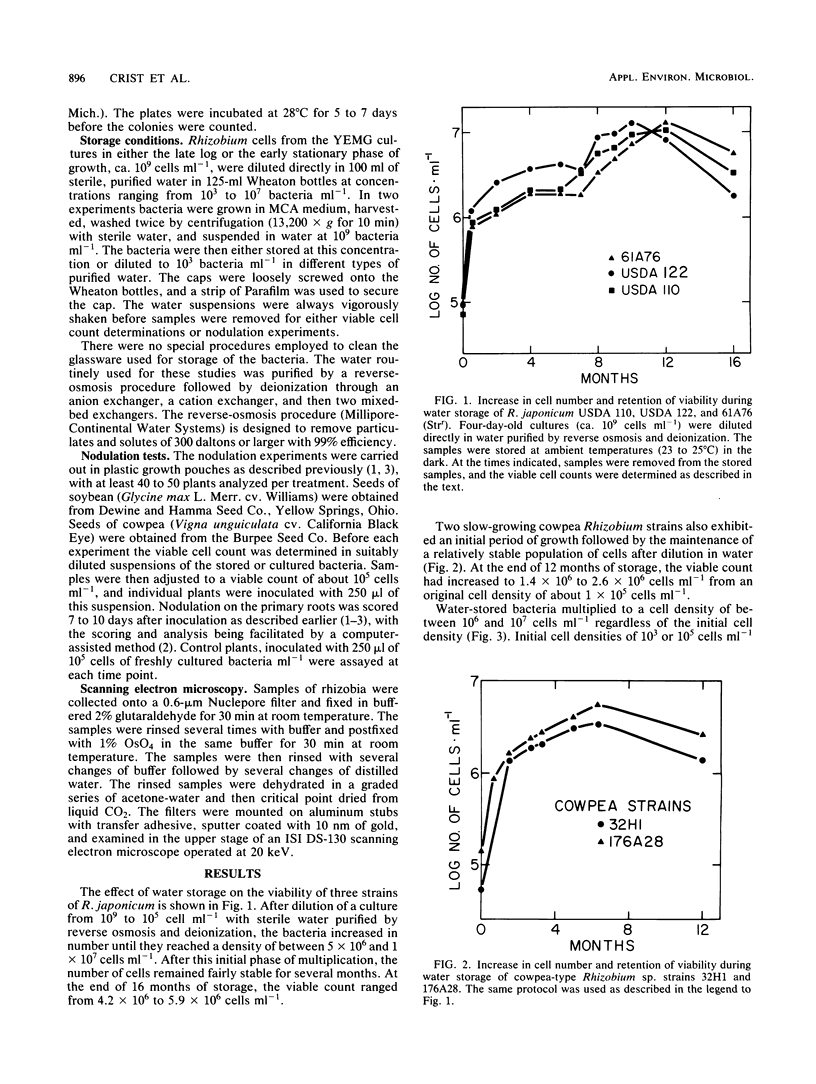
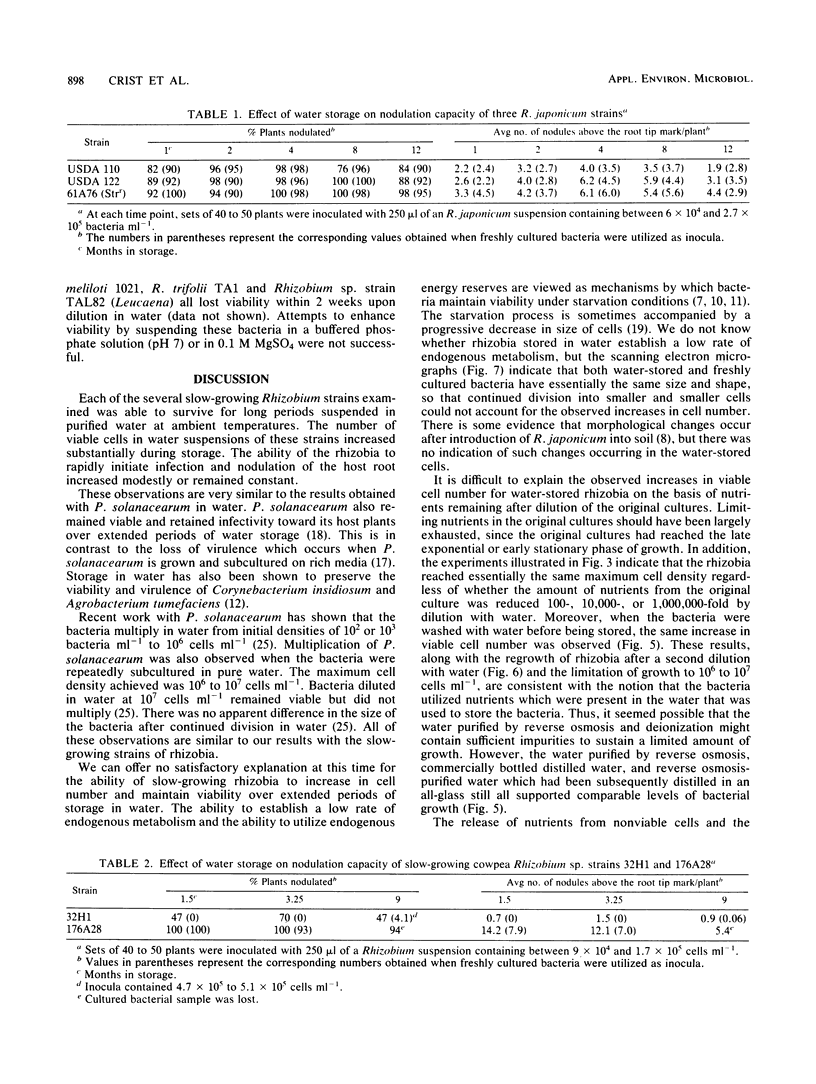
Three Rhizobium japonicum strains and two slow-growing cowpea-type Rhizobium strains were found to remain viable and able to rapidly modulate their respective hosts after being stored in purified water at ambient temperatures for periods of 1 year and longer. Three fast-growing Rhizobium species did not remain viable under the same water storage conditions. After dilution of slow-growing Rhizobium strains with water to 10(3) to 10(5) cells ml-1, the bacteria multiplied until the viable cell count reached levels of between 10(6) and 10(7) cells ml-1. The viable cell count subsequently remained fairly constant. When the rhizobia were diluted to 10(7) cells ml-1, they did not multiply, but full viability was maintained. If the rhizobia were washed and suspended at 10(9) cells ml-1, viability slowly declined to 10(7) cells ml-1 during 9 months of storage. Scanning electron microscopy showed that no major morphological changes took place during storage. Preservation of slow-growing rhizobia in water suspensions could provide a simple and inexpensive alternative to current methods for the preservation of rhizobia for legume inoculation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhuvaneswari T. V., Bhagwat A. A., Bauer W. D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981 Nov;68(5):1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Mills K. K., Crist D. K., Evans W. R., Bauer W. D. Effects of culture age on symbiotic infectivity of Rhizobium japonicum. J Bacteriol. 1983 Jan;153(1):443–451. doi: 10.1128/jb.153.1.443-451.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Turgeon B. G., Bauer W. D. Early Events in the Infection of Soybean (Glycine max L. Merr) by Rhizobium japonicum: I. LOCALIZATION OF INFECTIBLE ROOT CELLS. Plant Physiol. 1980 Dec;66(6):1027–1031. doi: 10.1104/pp.66.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton C. E. Aging of Pseudomonas aeruginosa. J Bacteriol. 1967 Dec;94(6):2077–2078. doi: 10.1128/jb.94.6.2077-2078.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVAY J. E., SCHNATHORST W. C. SINGLE-CELL ISOLATION AND PRESERVATION OF BACTERIAL CULTURES. Nature. 1963 Aug 24;199:775–777. doi: 10.1038/199775a0. [DOI] [PubMed] [Google Scholar]
- Dawes E. A., Senior P. J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- Druilhet R. E., Sobek J. M. Starvation survival of Salmonella enteritidis. J Bacteriol. 1976 Jan;125(1):119–124. doi: 10.1128/jb.125.1.119-124.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. R., Keister D. L. Reduction of acetylene by stationary cultures of free-living Rhizobium sp. under atmospheric oxygen levels. Can J Microbiol. 1976 Jul;22(7):949–952. doi: 10.1139/m76-137. [DOI] [PubMed] [Google Scholar]
- Kurath G., Morita R. Y. Starvation-Survival Physiological Studies of a Marine Pseudomonas sp. Appl Environ Microbiol. 1983 Apr;45(4):1206–1211. doi: 10.1128/aem.45.4.1206-1211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munévar F., Wollum A. G. Growth of Rhizobium japonicum Strains at Temperatures Above 27 degrees C. Appl Environ Microbiol. 1981 Aug;42(2):272–276. doi: 10.1128/aem.42.2.272-276.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson L. M., Parkinson D. Effect of starvation on survival of three bacterial isolates from an arctic soil. Can J Microbiol. 1978 Dec;24(12):1460–1467. doi: 10.1139/m78-235. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Somasegaran P., Halliday J. Dilution of liquid Rhizobium cultures to increase production capacity of inoculant plants. Appl Environ Microbiol. 1982 Aug;44(2):330–333. doi: 10.1128/aem.44.2.330-333.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



