Abstract
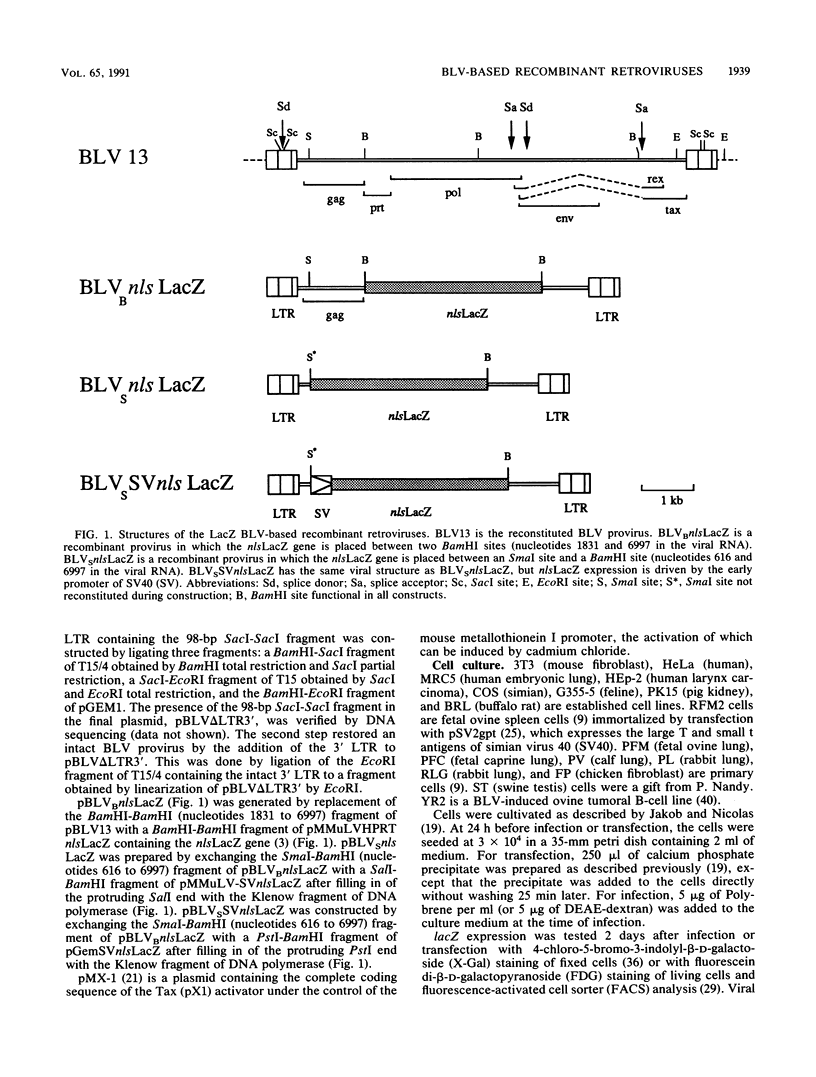
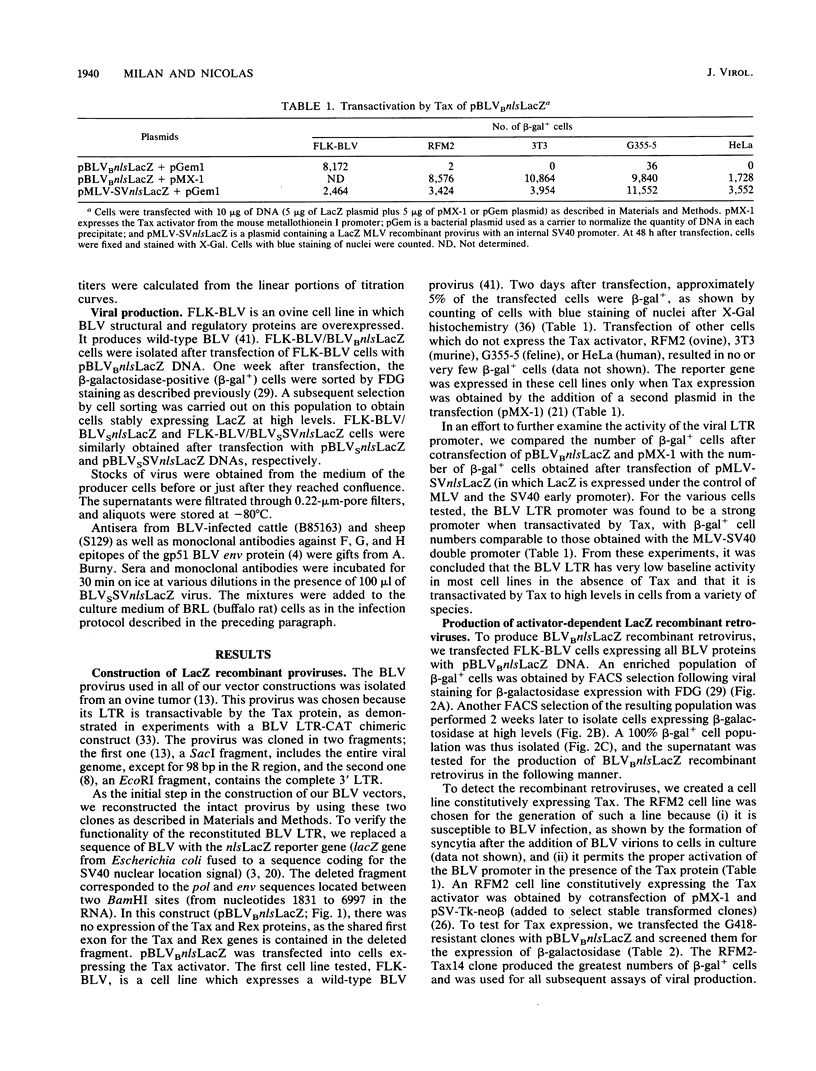
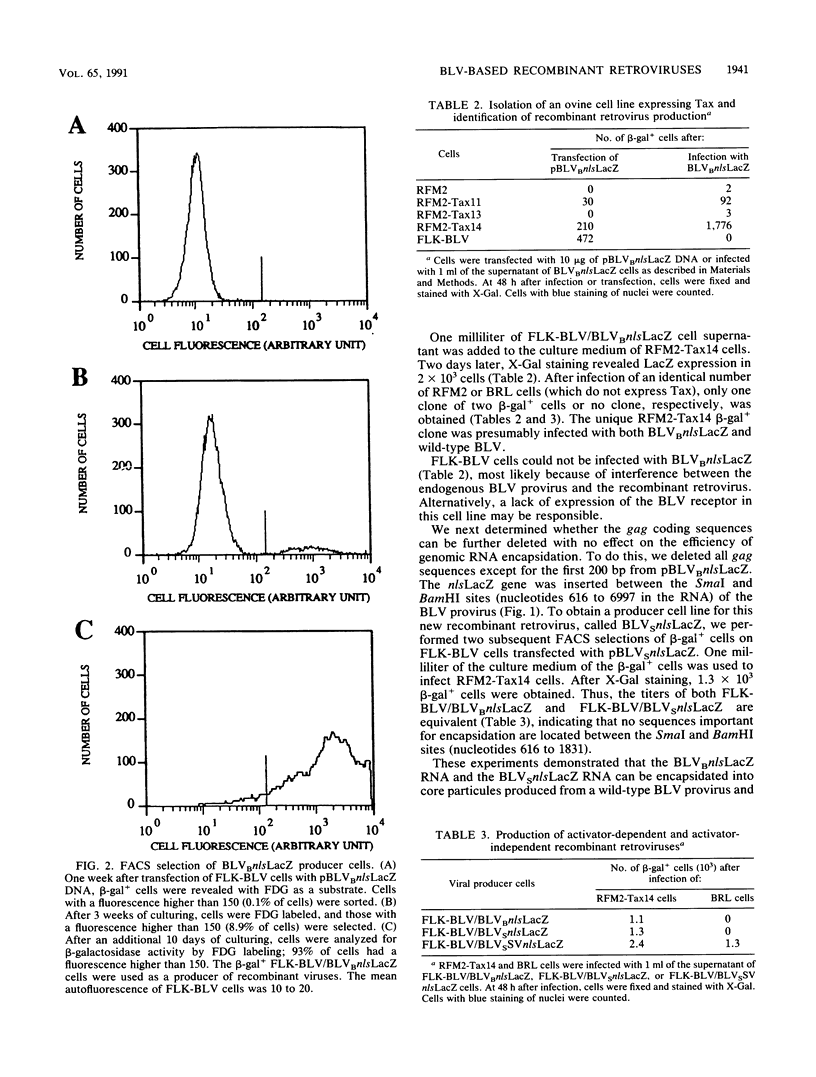
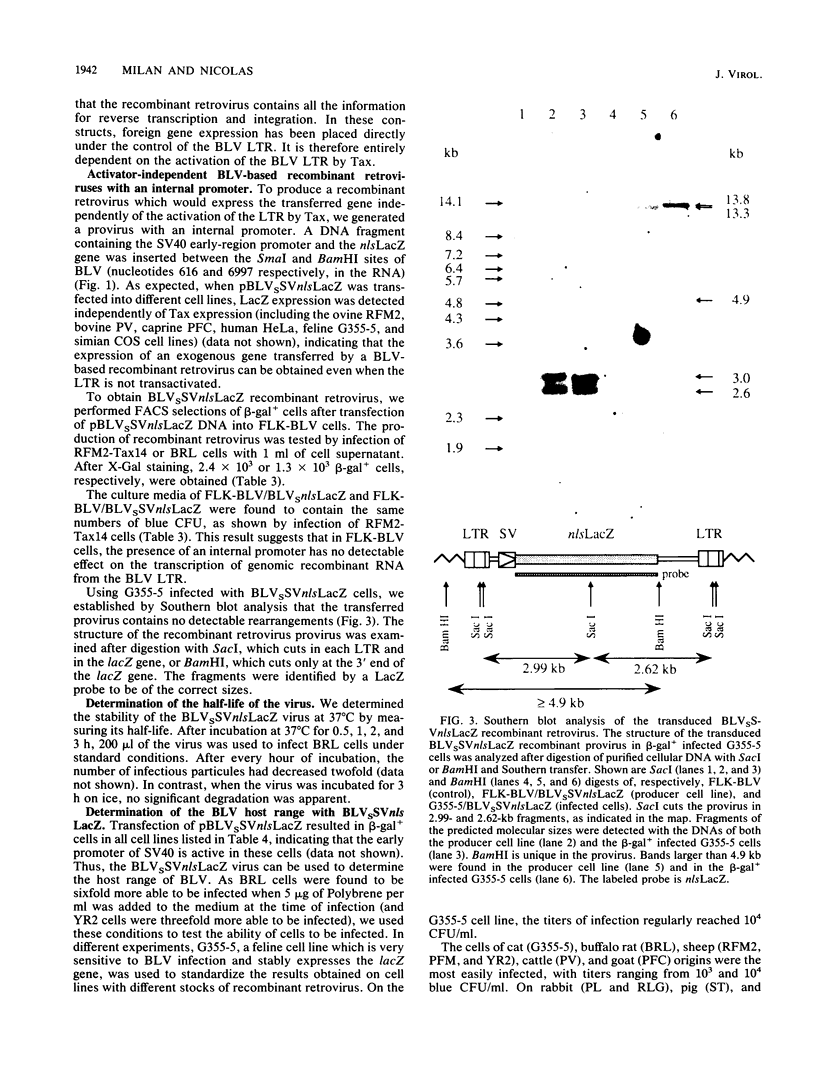
The replication-competent bovine leukemia virus (BLV) has been modified for use as a vector for foreign genes. The gag, pol, env, and pX regions of the virus were replaced by an exogenous nuclear location signal LacZ (nlsLacZ) or SVnlsLacZ gene. Transfection of the ovine cell line FLK-BLV, which expresses all BLV proteins from a wild-type provirus, with this viral DNA resulted in a viral titer of 10(4) CFU/ml. The inclusion of a large portion of the gag region did not significantly increase the titer. Both activator-dependent and activator-independent retroviruses were constructed. In activator-dependent vectors, the expression of the insert was dependent on the presence of the Tax protein, which activated the BLV long terminal repeat. In activator-independent vectors, the expression of the insert was constitutive because of the presence of an internal promoter. Infections with the recombinant retrovirus were inhibited by specific neutralizing antibodies. The structure of the transduced genetic material was not rearranged. BLV vectors encoding a reporter nlsLacZ gene, the product of which can be detected in single cells, greatly simplified studies of their biological properties. Determination of the host range of BLV vectors established that BLV-based recombinant retroviruses are effective in the transduction of genes in a variety of species and cell types.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ban J., First N. L., Temin H. M. Bovine leukaemia virus packaging cell line for retrovirus-mediated gene transfer. J Gen Virol. 1989 Aug;70(Pt 8):1987–1993. doi: 10.1099/0022-1317-70-8-1987. [DOI] [PubMed] [Google Scholar]
- Belmont J. W., Henkel-Tigges J., Chang S. M., Wager-Smith K., Kellems R. E., Dick J. E., Magli M. C., Phillips R. A., Bernstein A., Caskey C. T. Expression of human adenosine deaminase in murine haematopoietic progenitor cells following retroviral transfer. Nature. 1986 Jul 24;322(6077):385–387. doi: 10.1038/322385a0. [DOI] [PubMed] [Google Scholar]
- Bonnerot C., Rocancourt D., Briand P., Grimber G., Nicolas J. F. A beta-galactosidase hybrid protein targeted to nuclei as a marker for developmental studies. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6795–6799. doi: 10.1073/pnas.84.19.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Burny A., Zavada J. Topographical analysis by monoclonal antibodies of BLV-gp51 epitopes involved in viral functions. Virology. 1982 Oct 30;122(2):353–362. doi: 10.1016/0042-6822(82)90235-5. [DOI] [PubMed] [Google Scholar]
- Cone R. D., Mulligan R. C. High-efficiency gene transfer into mammalian cells: generation of helper-free recombinant retrovirus with broad mammalian host range. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6349–6353. doi: 10.1073/pnas.81.20.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone R. D., Reilly E. B., Eisen H. N., Mulligan R. C. Tissue-specific expression of functionally rearranged lambda 1 Ig gene through a retrovirus vector. Science. 1987 May 22;236(4804):954–957. doi: 10.1126/science.3107128. [DOI] [PubMed] [Google Scholar]
- Couez D., Deschamps J., Kettmann R., Stephens R. M., Gilden R. V., Burny A. Nucleotide sequence analysis of the long terminal repeat of integrated bovine leukemia provirus DNA and of adjacent viral and host sequences. J Virol. 1984 Feb;49(2):615–620. doi: 10.1128/jvi.49.2.615-620.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delouis C., Milan D., L'Haridon R., Gianquinto L., Bonnerot C., Nicolas J. F. Xenotropic and amphotropic pseudotyped recombinant retrovirus to transfer genes into cells from various species. Biochem Biophys Res Commun. 1990 May 31;169(1):8–14. doi: 10.1016/0006-291x(90)91425-r. [DOI] [PubMed] [Google Scholar]
- Derse D. Bovine leukemia virus transcription is controlled by a virus-encoded trans-acting factor and by cis-acting response elements. J Virol. 1987 Aug;61(8):2462–2471. doi: 10.1128/jvi.61.8.2462-2471.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D., Martarano L. Construction of a recombinant bovine leukemia virus vector for analysis of virus infectivity. J Virol. 1990 Jan;64(1):401–405. doi: 10.1128/jvi.64.1.401-405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derse D. trans-acting regulation of bovine leukemia virus mRNA processing. J Virol. 1988 Apr;62(4):1115–1119. doi: 10.1128/jvi.62.4.1115-1119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps J., Kettmann R., Burny A. Experiments with cloned complete tumor-derived bovine leukemia virus information prove that the virus is totally exogenous to its target animal species. J Virol. 1981 Nov;40(2):605–609. doi: 10.1128/jvi.40.2.605-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. A promoterless retroviral vector indicates that there are sequences in U3 required for 3' RNA processing. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1197–1201. doi: 10.1073/pnas.84.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature. 1986 Mar 20;320(6059):275–277. doi: 10.1038/320275a0. [DOI] [PubMed] [Google Scholar]
- Huszar D., Balling R., Kothary R., Magli M. C., Hozumi N., Rossant J., Bernstein A. Insertion of a bacterial gene into the mouse germ line using an infectious retrovirus vector. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8587–8591. doi: 10.1073/pnas.82.24.8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob H., Nicolas J. F. Mouse teratocarcinoma cells. Methods Enzymol. 1987;151:66–81. doi: 10.1016/s0076-6879(87)51009-6. [DOI] [PubMed] [Google Scholar]
- Jähner D., Haase K., Mulligan R., Jaenisch R. Insertion of the bacterial gpt gene into the germ line of mice by retroviral infection. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6927–6931. doi: 10.1073/pnas.82.20.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Sagata N., Ikawa Y. The bovine leukemia virus X region encodes a trans-activator of its long terminal repeat. Jpn J Cancer Res. 1987 Feb;78(2):93–98. [PubMed] [Google Scholar]
- Ledley F. D., Darlington G. J., Hahn T., Woo S. L. Retroviral gene transfer into primary hepatocytes: implications for genetic therapy of liver-specific functions. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5335–5339. doi: 10.1073/pnas.84.15.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Nicolas J. F., Rubenstein J. L., Bonnerot C., Jacob F. Introduction of genes into embryonal carcinoma cells and preimplantation embryos by retroviral vectors. Cold Spring Harb Symp Quant Biol. 1985;50:713–720. doi: 10.1101/sqb.1985.050.01.088. [DOI] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocancourt D., Bonnerot C., Jouin H., Emerman M., Nicolas J. F. Activation of a beta-galactosidase recombinant provirus: application to titration of human immunodeficiency virus (HIV) and HIV-infected cells. J Virol. 1990 Jun;64(6):2660–2668. doi: 10.1128/jvi.64.6.2660-2668.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Sodroski J. G., Kettman R., Burny A., Haseltine W. A. Trans activation of the bovine leukemia virus long terminal repeat in BLV-infected cells. Science. 1985 Jan 18;227(4684):320–322. doi: 10.1126/science.2981432. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. Construction of a retrovirus capable of transducing and expressing genes in multipotential embryonic cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7137–7140. doi: 10.1073/pnas.81.22.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagata N., Yasunaga T., Tsuzuku-Kawamura J., Ohishi K., Ogawa Y., Ikawa Y. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci U S A. 1985 Feb;82(3):677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatier N., Rocancourt D., Bonnerot C., Nicolas J. F. A novel system for screening antiretroviral agents. J Virol Methods. 1989 Nov;26(2):229–235. doi: 10.1016/0166-0934(89)90153-5. [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Rüther U., Garber C., Vanek M., Wagner E. F. The expression of retroviral vectors in murine stem cells and transgenic mice. J Embryol Exp Morphol. 1986 Oct;97 (Suppl):263–275. [PubMed] [Google Scholar]
- Stuhlmann H., Jaenisch R., Mulligan R. C. Transfer of a mutant dihydrofolate reductase gene into pre- and postimplantation mouse embryos by a replication-competent retrovirus vector. J Virol. 1989 Nov;63(11):4857–4865. doi: 10.1128/jvi.63.11.4857-4865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeke A., Cleuter Y., Chen G., Portetelle D., Mammerickx M., Zagury D., Fouchard M., Coulombel L., Kettmann R., Burny A. Even transcriptionally competent proviruses are silent in bovine leukemia virus-induced sheep tumor cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9263–9267. doi: 10.1073/pnas.85.23.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Vanek M., Vennström B. Transfer of genes into embryonal carcinoma cells by retrovirus infection: efficient expression from an internal promoter. EMBO J. 1985 Mar;4(3):663–666. doi: 10.1002/j.1460-2075.1985.tb03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. F., von Rüden T., Kantoff P. W., Garber C., Seiberg M., Rüther U., Anderson W. F., Wagner E. F., Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]



