Abstract
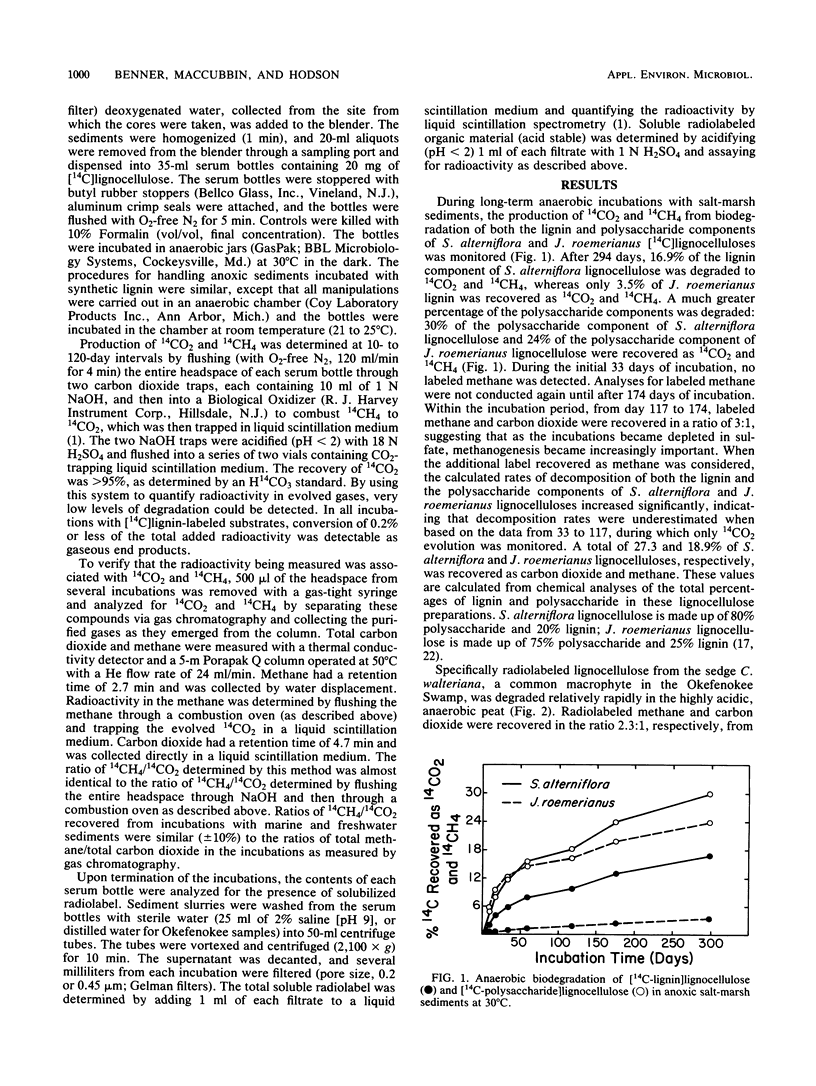
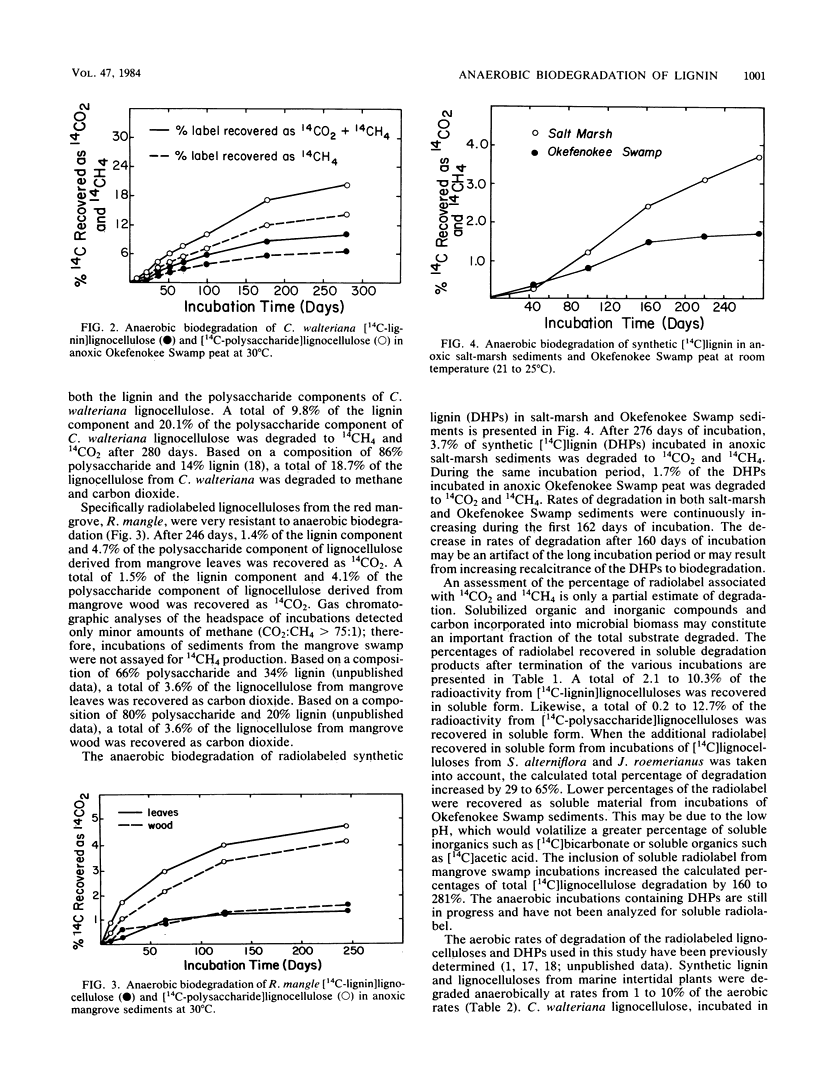
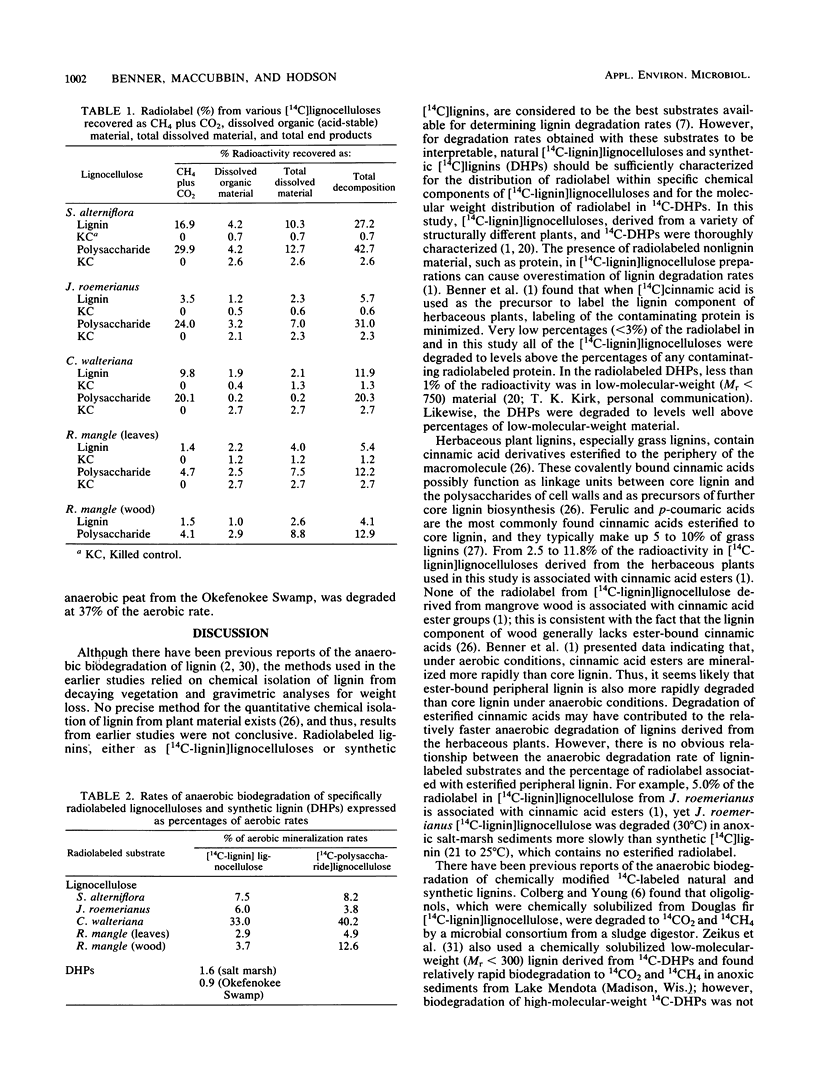
Specifically radiolabeled [14C-lignin]lignocelluloses and [14C-polysaccharide]lignocelluloses were prepared from a variety of marine and freshwater wetland plants including a grass, a sedge, a rush, and a hardwood. These [14C]lignocellulose preparations and synthetic [14C]lignin were incubated anaerobically with anoxic sediments collected from a salt marsh, a freshwater marsh, and a mangrove swamp. During long-term incubations lasting up to 300 days, the lignin and polysaccharide components of the lignocelluloses were slowly degraded anaerobically to 14CO2 and 14CH4. Lignocelluloses derived from herbaceous plants were degraded more rapidly than lignocellulose derived from the hardwood. After 294 days, 16.9% of the lignin component and 30.0% of the polysaccharide component of lignocellulose derived from the grass used (Spartina alterniflora) were degraded to gaseous end products. In contrast, after 246 days, only 1.5% of the lignin component and 4.1% of the polysaccharide component of lignocellulose derived from the hardwood used (Rhizophora mangle) were degraded to gaseous end products. Synthetic [14C]lignin was degraded anaerobically faster than the lignin component of the hardwood lignocellulose; after 276 days, 3.7% of the synthetic lignin was degraded to gaseous end products. Contrary to previous reports, these results demonstrate that lignin and lignified plant tissues are biodegradable in the absence of oxygen. Although lignocelluloses are recalcitrant to anaerobic biodegradation, rates of degradation measured in aquatic sediments are significant and have important implications for the biospheric cycling of carbon from these abundant biopolymers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benner R., Maccubbin A. E., Hodson R. E. Preparation, characterization, and microbial degradation of specifically radiolabeled [C]lignocelluloses from marine and freshwater macrophytes. Appl Environ Microbiol. 1984 Feb;47(2):381–389. doi: 10.1128/aem.47.2.381-389.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C. Biochemistry of the bacterial catabolism of aromatic compounds in anaerobic environments. Nature. 1977 Nov 3;270(5632):17–22. doi: 10.1038/270017a0. [DOI] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Relationship Between Lignin Degradation and Production of Reduced Oxygen Species by Phanerochaete chrysosporium. Appl Environ Microbiol. 1983 Nov;46(5):1140–1145. doi: 10.1128/aem.46.5.1140-1145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney L. J., Reddy C. A., Tien M., Aust S. D. The involvement of hydroxyl radical derived from hydrogen peroxide in lignin degradation by the white rot fungus Phanerochaete chrysosporium. J Biol Chem. 1982 Oct 10;257(19):11455–11462. [PubMed] [Google Scholar]
- Hackett W. F., Connors W. J., Kirk T. K., Zeikus J. G. Microbial decomposition of synthetic C-labeled lignins in nature: lignin biodegradation in a variety of natural materials. Appl Environ Microbiol. 1977 Jan;33(1):43–51. doi: 10.1128/aem.33.1.43-51.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy J. B., Young L. Y. Anaerobic biodegradation of eleven aromatic compounds to methane. Appl Environ Microbiol. 1979 Jul;38(1):84–89. doi: 10.1128/aem.38.1.84-89.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy J. B., Young L. Y., Reinhard M. Methanogenic decomposition of ferulic Acid, a model lignin derivative. Appl Environ Microbiol. 1980 Feb;39(2):436–444. doi: 10.1128/aem.39.2.436-444.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Bleam R. D., Hackett W. F., Zeikus J. G. Preparation and microbial decomposition of synthetic [14C]ligins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2515–2519. doi: 10.1073/pnas.72.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccubbin A. E., Hodson R. E. Mineralization of detrital lignocelluloses by salt marsh sediment microflora. Appl Environ Microbiol. 1980 Oct;40(4):735–740. doi: 10.1128/aem.40.4.735-740.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odier E., Monties B. Absence of microbial mineralization of lignin in anaerobic enrichment cultures. Appl Environ Microbiol. 1983 Sep;46(3):661–665. doi: 10.1128/aem.46.3.661-665.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan A. J., Pippenger C. E., McGregor P. A., French J. H. Seizure activity and anticonvulsant drug concentration. Arch Neurol. 1975 May;32(5):281–288. doi: 10.1001/archneur.1975.00490470025002. [DOI] [PubMed] [Google Scholar]
- Taylor B. F. Aerobic and Anaerobic Catabolism of Vanillic Acid and Some Other Methoxy-Aromatic Compounds by Pseudomonas sp. Strain PN-1. Appl Environ Microbiol. 1983 Dec;46(6):1286–1292. doi: 10.1128/aem.46.6.1286-1292.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Heeb M. J. The anaerobic degradation of aromatic compounds by a denitrifying bacterium. Radioisotope and mutant studies. Arch Mikrobiol. 1972;83(2):165–171. doi: 10.1007/BF00425023. [DOI] [PubMed] [Google Scholar]


