Abstract
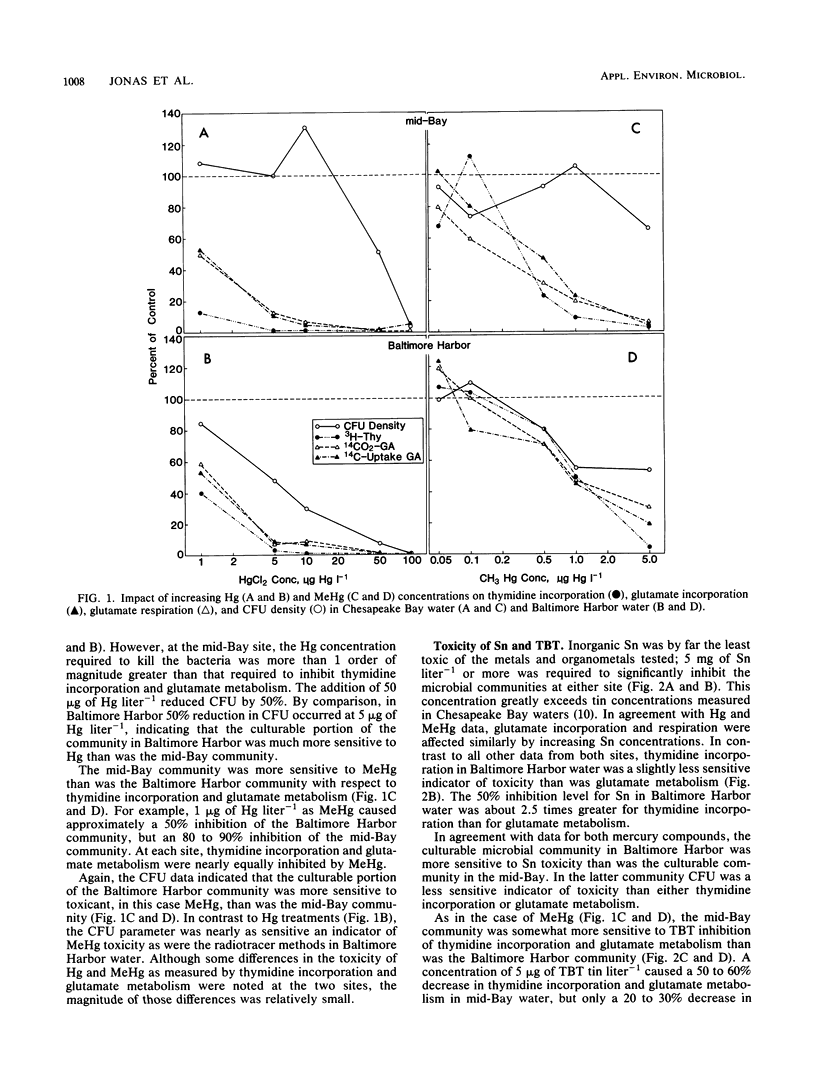
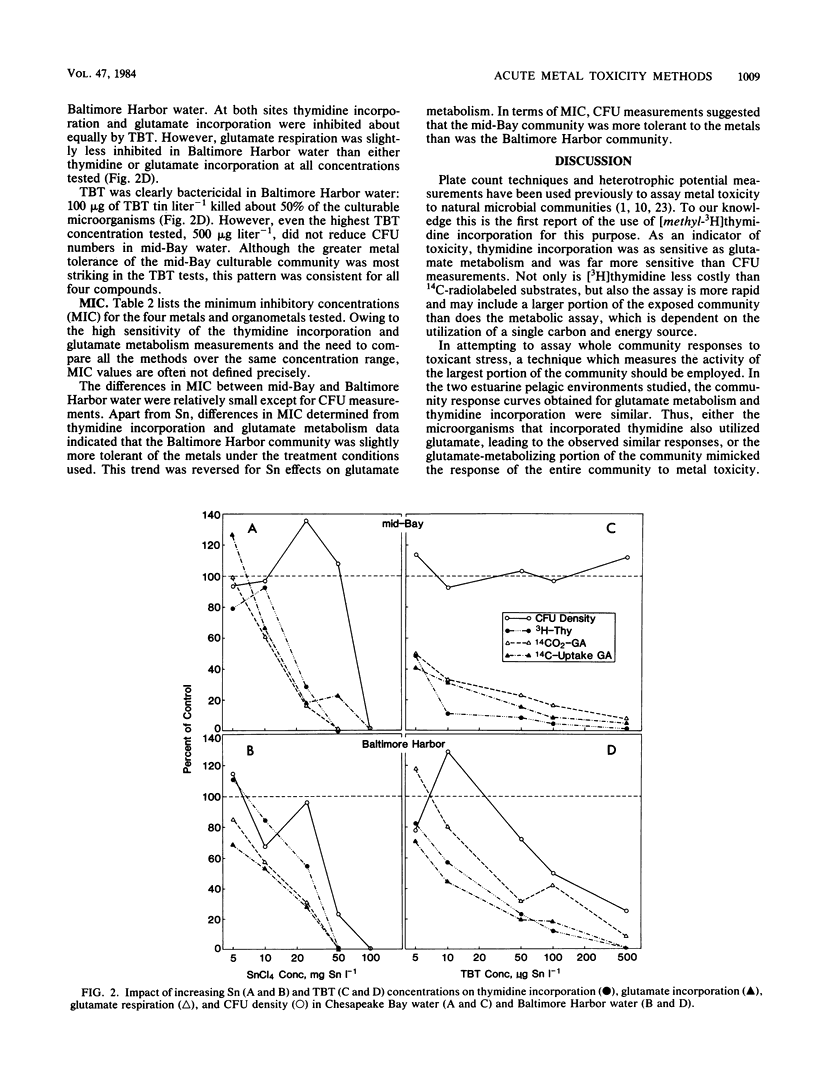
Microbial communities in water from Baltimore Harbor and from the mainstem of Chesapeake Bay were examined for sensitivity to mercuric chloride, monomethyl mercury, stannic chloride, and tributyltin chloride. Acute toxicity was determined by measuring the effects of [3H]thymidine incorporation, [14C]glutamate incorporation and respiration, and viability as compared with those of controls. Minimum inhibitory concentrations were low for all metals (monomethyl mercury, less than 0.05 microgram liter-1; mercuric chloride, less than 1 microgram liter-1; tributyltin chloride, less than 5 micrograms liter-1) except stannic chloride (5 mg liter-1). In some cases, mercuric chloride and monomethyl mercury were equally toxic at comparable concentrations. The Chesapeake Bay community appeared to be slightly more sensitive to metal stress than the Baltimore Harbor community, but this was not true for all treatments or assays. For culturable bacteria the opposite result was found. Thymidine incorporation and glutamate metabolism were much more sensitive indicators of metal toxicity than was viability. To our knowledge, this is the first use of the thymidine incorporation method for ecotoxicology studies. We found it the easiest and fastest of the three methods; it is at least equal in sensitivity to metabolic measurements, and it likely measures the effects on greater portion of the natural community.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fuhrman J. A., Azam F. Bacterioplankton secondary production estimates for coastal waters of british columbia, antarctica, and california. Appl Environ Microbiol. 1980 Jun;39(6):1085–1095. doi: 10.1128/aem.39.6.1085-1095.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths R. P., Hanus F. J., Morita R. Y. The effects of various water-sample treatments on the apparent uptake of glutamic acid by natural marine microbial populations. Can J Microbiol. 1974 Sep;20(9):1261–1266. doi: 10.1139/m74-194. [DOI] [PubMed] [Google Scholar]
- Hallas L. E., Cooney J. J. Tin and tin-resistant microorganisms in chesapeake bay. Appl Environ Microbiol. 1981 Feb;41(2):466–471. doi: 10.1128/aem.41.2.466-471.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Hou H. S., Imura N. Physiological role of mercury-methylation in Clostridium cochlearium T-2C. Bull Environ Contam Toxicol. 1982 Sep;29(3):290–297. doi: 10.1007/BF01706231. [DOI] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. The action of tributyltin chloride on the uptake of proline and glutamine by intact cells of Escherichia coli. Can J Biochem. 1979 Dec;57(12):1376–1383. doi: 10.1139/o79-183. [DOI] [PubMed] [Google Scholar]
- Soracco R. J., Pope D. H. Bacteriostatic and bactericidal modes of action of bis(tributyltin)oxide on Legionella pneumophila. Appl Environ Microbiol. 1983 Jan;45(1):48–57. doi: 10.1128/aem.45.1.48-57.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle J. H. Organic carbon utilization by resting cells of thiosulfate-oxidizing marine heterotrophs. Appl Environ Microbiol. 1980 Sep;40(3):516–521. doi: 10.1128/aem.40.3.516-521.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]


