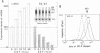Abstract
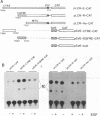
We have found that epidermal growth factor (EGF) elicits negative regulation of human papillomavirus type 16 (HPV-16) E6/E7 at the mRNA level in the HPV-16-immortalized human keratinocyte cell line (PHK160b). This down-regulation of HPV-16 E6/E7 expression was achieved when the cells were stimulated to proliferate with the concomitantly enhanced c-myc expression by EGF in a dose-dependent manner. By using partly synchronized PHK160b cells, negative and positive regulations of the HPV-16 E6/E7 expression was correlated to EGF-linked cell cycle events in this particular human keratinocyte cell line. In order to study transcriptional control mechanisms of the HPV-16 E6/E7, transient expression assays were performed with CAT expression plasmids that the transcription could be directed by the 5'-deleted HPV-16 long control region (LCR) including the virus P97 promoter. We demonstrated that the HPV-16 LCR contained EGF-responsive elements and that a predominant silencer activity was mapped in the proximal 124-bp region (EGFRE) of the LCR. This restricted LCR region had significant influence on HPV-16-homologous promoters in lowering the CAT expression in the presence and absence of EGF. EGFRE was thus considered to be a conditional transcription-controlling element on HPV-16 E6/E7 expression in this EGF-responsive human keratinocyte cell line. This suggests that specific sequences in the LCR play a critical part in the EGF-induced down-regulation of E6/E7 expression at the transcriptional level. Since the results obtained from the transient expression assay agreed with the mode of expression of the endogenous HPV-16 E6/E7, the present study strongly suggests that the transcriptional regulation of the HPV-16 E6/E7 oncogene is mediated by growth-related specific cellular factors interacting with HPV-16 LCR elements.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Androphy E. J., Lowy D. R., Schiller J. T. Bovine papillomavirus E2 trans-activating gene product binds to specific sites in papillomavirus DNA. Nature. 1987 Jan 1;325(6099):70–73. doi: 10.1038/325070a0. [DOI] [PubMed] [Google Scholar]
- Barrandon Y., Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987 Sep 25;50(7):1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Berg P. E., Popovic Z., Anderson W. F. Promoter dependence of enhancer activity. Mol Cell Biol. 1984 Aug;4(8):1664–1668. doi: 10.1128/mcb.4.8.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing M., Zentgraf H., Jorcano J. L. Differentially expressed bovine cytokeratin genes. Analysis of gene linkage and evolutionary conservation of 5'-upstream sequences. EMBO J. 1987 Mar;6(3):567–575. doi: 10.1002/j.1460-2075.1987.tb04792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce S. T., Ham R. G. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983 Jul;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- Braun L., Dürst M., Mikumo R., Gruppuso P. Differential response of nontumorigenic and tumorigenic human papillomavirus type 16-positive epithelial cells to transforming growth factor beta 1. Cancer Res. 1990 Nov 15;50(22):7324–7332. [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Stimulation and inhibition of growth by EGF in different A431 cell clones is accompanied by the rapid induction of c-fos and c-myc proto-oncogenes. EMBO J. 1985 May;4(5):1193–1197. doi: 10.1002/j.1460-2075.1985.tb03759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G. Receptors for epidermal growth factor and other polypeptide mitogens. Annu Rev Biochem. 1987;56:881–914. doi: 10.1146/annurev.bi.56.070187.004313. [DOI] [PubMed] [Google Scholar]
- Chan W. K., Chong T., Bernard H. U., Klock G. Transcription of the transforming genes of the oncogenic human papillomavirus-16 is stimulated by tumor promotors through AP1 binding sites. Nucleic Acids Res. 1990 Feb 25;18(4):763–769. doi: 10.1093/nar/18.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan W. K., Klock G., Bernard H. U. Progesterone and glucocorticoid response elements occur in the long control regions of several human papillomaviruses involved in anogenital neoplasia. J Virol. 1989 Aug;63(8):3261–3269. doi: 10.1128/jvi.63.8.3261-3269.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin M. T., Hirochika R., Hirochika H., Broker T. R., Chow L. T. Regulation of human papillomavirus type 11 enhancer and E6 promoter by activating and repressing proteins from the E2 open reading frame: functional and biochemical studies. J Virol. 1988 Aug;62(8):2994–3002. doi: 10.1128/jvi.62.8.2994-3002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong T., Chan W. K., Bernard H. U. Transcriptional activation of human papillomavirus 16 by nuclear factor I, AP1, steroid receptors and a possibly novel transcription factor, PVF: a model for the composition of genital papillomavirus enhancers. Nucleic Acids Res. 1990 Feb 11;18(3):465–470. doi: 10.1093/nar/18.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Cripe T. P., Alderborn A., Anderson R. D., Parkkinen S., Bergman P., Haugen T. H., Pettersson U., Turek L. P. Transcriptional activation of the human papillomavirus-16 P97 promoter by an 88-nucleotide enhancer containing distinct cell-dependent and AP-1-responsive modules. New Biol. 1990 May;2(5):450–463. [PubMed] [Google Scholar]
- Cripe T. P., Haugen T. H., Turk J. P., Tabatabai F., Schmid P. G., 3rd, Dürst M., Gissmann L., Roman A., Turek L. P. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. EMBO J. 1987 Dec 1;6(12):3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore P. P., Falco J., Borrello I., Weissman B., Aaronson S. A. The calcium signal for BALB/MK keratinocyte terminal differentiation counteracts epidermal growth factor (EGF) very early in the EGF-induced proliferative pathway. Mol Cell Biol. 1988 Feb;8(2):557–563. doi: 10.1128/mcb.8.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Dzarlieva-Petrusevska R. T., Boukamp P., Fusenig N. E., Gissmann L. Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene. 1987;1(3):251–256. [PubMed] [Google Scholar]
- Filmus J., Benchimol S., Buick R. N. Comparative analysis of the involvement of p53, c-myc and c-fos in epidermal growth factor-mediated signal transduction. Exp Cell Res. 1987 Apr;169(2):554–559. doi: 10.1016/0014-4827(87)90215-1. [DOI] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Roeder R. G. An AP1-binding site in the c-fos gene can mediate induction by epidermal growth factor and 12-O-tetradecanoyl phorbol-13-acetate. Mol Cell Biol. 1989 Mar;9(3):1327–1331. doi: 10.1128/mcb.9.3.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Carranca A., Thierry F., Yaniv M. Interplay of viral and cellular proteins along the long control region of human papillomavirus type 18. J Virol. 1988 Nov;62(11):4321–4330. doi: 10.1128/jvi.62.11.4321-4330.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss B., Bernard H. U., Seedorf K., Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987 Dec 1;6(12):3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss B., Chong T., Bernard H. U. Numerous nuclear proteins bind the long control region of human papillomavirus type 16: a subset of 6 of 23 DNase I-protected segments coincides with the location of the cell-type-specific enhancer. J Virol. 1989 Mar;63(3):1142–1152. doi: 10.1128/jvi.63.3.1142-1152.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloss B., Yeo-Gloss M., Meisterenst M., Rogge L., Winnacker E. L., Bernard H. U. Clusters of nuclear factor I binding sites identify enhancers of several papillomaviruses but alone are not sufficient for enhancer function. Nucleic Acids Res. 1989 May 11;17(9):3519–3533. doi: 10.1093/nar/17.9.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Couchman J. R. Differences in human skin between the epidermal growth factor receptor distribution detected by EGF binding and monoclonal antibody recognition. J Invest Dermatol. 1985 Sep;85(3):239–245. doi: 10.1111/1523-1747.ep12276708. [DOI] [PubMed] [Google Scholar]
- Hashida T., Yasumoto S. Casein kinase II activities related to hyperphosphorylation of human papillomavirus type 16-E7 oncoprotein in epidermal keratinocytes. Biochem Biophys Res Commun. 1990 Oct 30;172(2):958–964. doi: 10.1016/0006-291x(90)90769-j. [DOI] [PubMed] [Google Scholar]
- Haugen T. H., Cripe T. P., Ginder G. D., Karin M., Turek L. P. Trans-activation of an upstream early gene promoter of bovine papilloma virus-1 by a product of the viral E2 gene. EMBO J. 1987 Jan;6(1):145–152. doi: 10.1002/j.1460-2075.1987.tb04732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen T. H., Turek L. P., Mercurio F. M., Cripe T. P., Olson B. J., Anderson R. D., Seidl D., Karin M., Schiller J. Sequence-specific and general transcriptional activation by the bovine papillomavirus-1 E2 trans-activator require an N-terminal amphipathic helix-containing E2 domain. EMBO J. 1988 Dec 20;7(13):4245–4253. doi: 10.1002/j.1460-2075.1988.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Broker T. R., Chow L. T. Enhancers and trans-acting E2 transcriptional factors of papillomaviruses. J Virol. 1987 Aug;61(8):2599–2606. doi: 10.1128/jvi.61.8.2599-2606.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. K., Nørgård J. O., Bolund L. Changes in basal cell subpopulations and tissue differentiation in human epidermal cultures treated with epidermal growth factor and cholera toxin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1985;49(4):325–340. doi: 10.1007/BF02912110. [DOI] [PubMed] [Google Scholar]
- Kleiner E., Dietrich W., Pfister H. Differential regulation of papilloma virus early gene expression in transformed fibroblasts and carcinoma cell lines. EMBO J. 1986 Aug;5(8):1945–1950. doi: 10.1002/j.1460-2075.1986.tb04448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koi M., Morita H., Yamada H., Satoh H., Barrett J. C., Oshimura M. Normal human chromosome 11 suppresses tumorigenicity of human cervical tumor cell line SiHa. Mol Carcinog. 1989;2(1):12–21. doi: 10.1002/mc.2940020103. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Spalholz B. A., Howley P. M. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987 Jul 3;50(1):69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Liboi E., Pelosi E., Testa U., Peschle C., Rossi G. B. Proliferative response and oncogene expression induced by epidermal growth factor in EL2 rat fibroblasts. Mol Cell Biol. 1986 Jun;6(6):2275–2278. doi: 10.1128/mcb.6.6.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Vass W. C., Schiller J. T., Lowy D. R., Velu T. J. The bovine papillomavirus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell. 1989 Oct 6;59(1):21–32. doi: 10.1016/0092-8674(89)90866-0. [DOI] [PubMed] [Google Scholar]
- McBride A. A., Schlegel R., Howley P. M. The carboxy-terminal domain shared by the bovine papillomavirus E2 transactivator and repressor proteins contains a specific DNA binding activity. EMBO J. 1988 Feb;7(2):533–539. doi: 10.1002/j.1460-2075.1988.tb02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey P., Ran W., Campisi J., Rosner M. R. Two independent growth factor-generated signals regulate c-fos and c-myc mRNA levels in Swiss 3T3 cells. J Biol Chem. 1987 Feb 5;262(4):1442–1445. [PubMed] [Google Scholar]
- McCance D. J., Kopan R., Fuchs E., Laimins L. A. Human papillomavirus type 16 alters human epithelial cell differentiation in vitro. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7169–7173. doi: 10.1073/pnas.85.19.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pater M. M., Hughes G. A., Hyslop D. E., Nakshatri H., Pater A. Glucocorticoid-dependent oncogenic transformation by type 16 but not type 11 human papilloma virus DNA. Nature. 1988 Oct 27;335(6193):832–835. doi: 10.1038/335832a0. [DOI] [PubMed] [Google Scholar]
- Pirisi L., Yasumoto S., Feller M., Doniger J., DiPaolo J. A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran W., Dean M., Levine R. A., Henkle C., Campisi J. Induction of c-fos and c-myc mRNA by epidermal growth factor or calcium ionophore is cAMP dependent. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8216–8220. doi: 10.1073/pnas.83.21.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977 Feb 3;265(5593):421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Romanczuk H., Thierry F., Howley P. M. Mutational analysis of cis elements involved in E2 modulation of human papillomavirus type 16 P97 and type 18 P105 promoters. J Virol. 1990 Jun;64(6):2849–2859. doi: 10.1128/jvi.64.6.2849-2859.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösl F., Dürst M., zur Hausen H. Selective suppression of human papillomavirus transcription in non-tumorigenic cells by 5-azacytidine. EMBO J. 1988 May;7(5):1321–1328. doi: 10.1002/j.1460-2075.1988.tb02947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon P. J., Srivatsan E. S., Stanbridge E. J. Introduction of human chromosome 11 via microcell transfer controls tumorigenic expression of HeLa cells. EMBO J. 1986 Dec 20;5(13):3461–3466. doi: 10.1002/j.1460-2075.1986.tb04670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Phelps W. C., Zhang Y. L., Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J. 1988 Oct;7(10):3181–3187. doi: 10.1002/j.1460-2075.1988.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J Cell Biol. 1986 Dec;103(6 Pt 1):2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasawa H., Tomita Y., Kubota K., Kasai T., Sekiya S., Takamizawa H., Simizu B. Transcriptional differences of the human papillomavirus type 16 genome between precancerous lesions and invasive carcinomas. J Virol. 1988 Mar;62(3):1022–1027. doi: 10.1128/jvi.62.3.1022-1027.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smotkin D., Wettstein F. O. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4680–4684. doi: 10.1073/pnas.83.13.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Roberts A. B., Roche N. S., Sporn M. B., Weinberg R. A. Differential responsiveness of myc- and ras-transfected cells to growth factors: selective stimulation of myc-transfected cells by epidermal growth factor. Mol Cell Biol. 1986 Mar;6(3):870–877. doi: 10.1128/mcb.6.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift F. V., Bhat K., Younghusband H. B., Hamada H. Characterization of a cell type-specific enhancer found in the human papilloma virus type 18 genome. EMBO J. 1987 May;6(5):1339–1344. doi: 10.1002/j.1460-2075.1987.tb02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi A., Yasumoto S. A major transcript of human papillomavirus type 16 in transformed NIH 3T3 cells contains polycistronic mRNA encoding E7, E5, and E1--E4 fusion gene. Virus Genes. 1990 Feb;3(3):221–233. doi: 10.1007/BF00393182. [DOI] [PubMed] [Google Scholar]
- Thierry F., Yaniv M. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 1987 Nov;6(11):3391–3397. doi: 10.1002/j.1460-2075.1987.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefald F. C., Devlin B. H., Williams R. S. Functional heterogeneity of mammalian TATA-box sequences revealed by interaction with a cell-specific enhancer. Nature. 1990 Mar 15;344(6263):260–262. doi: 10.1038/344260a0. [DOI] [PubMed] [Google Scholar]
- Wilke M. S., Hsu B. M., Wille J. J., Jr, Pittelkow M. R., Scott R. E. Biologic mechanisms for the regulation of normal human keratinocyte proliferation and differentiation. Am J Pathol. 1988 Apr;131(1):171–181. [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Bowden P. E., Doniger J., Pirisi L., Barnes W., Lancaster W. D., DiPaolo J. A. Characterization of normal human exocervical epithelial cells immortalized in vitro by papillomavirus types 16 and 18 DNA. Cancer Res. 1988 Aug 15;48(16):4620–4628. [PubMed] [Google Scholar]
- Woodworth C. D., Doniger J., DiPaolo J. A. Immortalization of human foreskin keratinocytes by various human papillomavirus DNAs corresponds to their association with cervical carcinoma. J Virol. 1989 Jan;63(1):159–164. doi: 10.1128/jvi.63.1.159-164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C. D., Notario V., DiPaolo J. A. Transforming growth factors beta 1 and 2 transcriptionally regulate human papillomavirus (HPV) type 16 early gene expression in HPV-immortalized human genital epithelial cells. J Virol. 1990 Oct;64(10):4767–4775. doi: 10.1128/jvi.64.10.4767-4775.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto S., Doniger J., DiPaolo J. A. Differential early viral gene expression in two stages of human papillomavirus type 16 DNA-induced malignant transformation. Mol Cell Biol. 1987 Jun;7(6):2165–2172. doi: 10.1128/mcb.7.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]