Abstract
Exposure of Escherichia coli to 3 microM Cd2+ results in 84 to 95% of the cells losing their ability to form colonies on plates of nutrient agar. Transfer of the cells to Cd2+-free liquid medium results in a recovery of colony-forming ability without significant synthesis of DNA. As an early event in recovery, the cells exhibit a rapid uptake of [3H]leucine. Recovery and this incorporation are inhibited by chloramphenicol or rifampin. Sodium dodecyl sulfate-gel electrophoresis of proteins from recovering cells labeled with [3H]leucine for 1 min indicated the synthesis of at least two classes of proteins with apparent molecular weights of 55,000 to 65,000. One class bound Cd2+ and was absent in untreated cultures. The other class of proteins, which did not bind Cd2+, was synthesized at a rapid rate in recovering cells and may be a normal cellular protein.
Full text
PDF
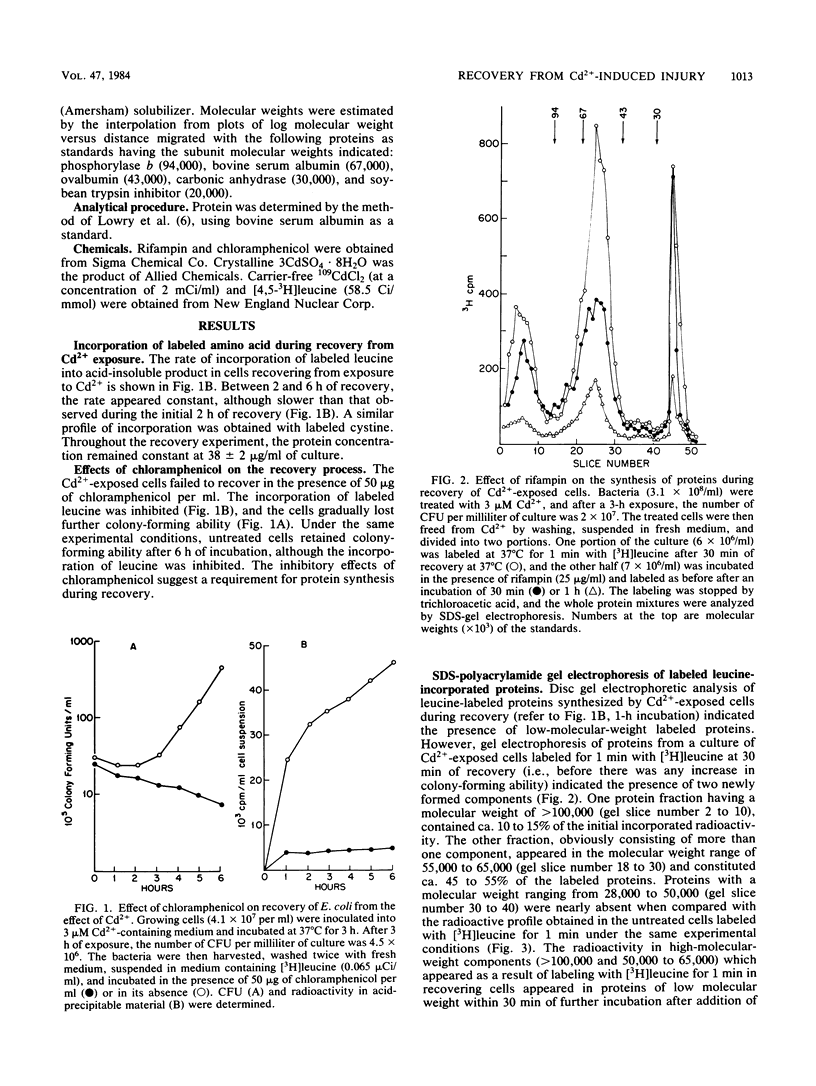
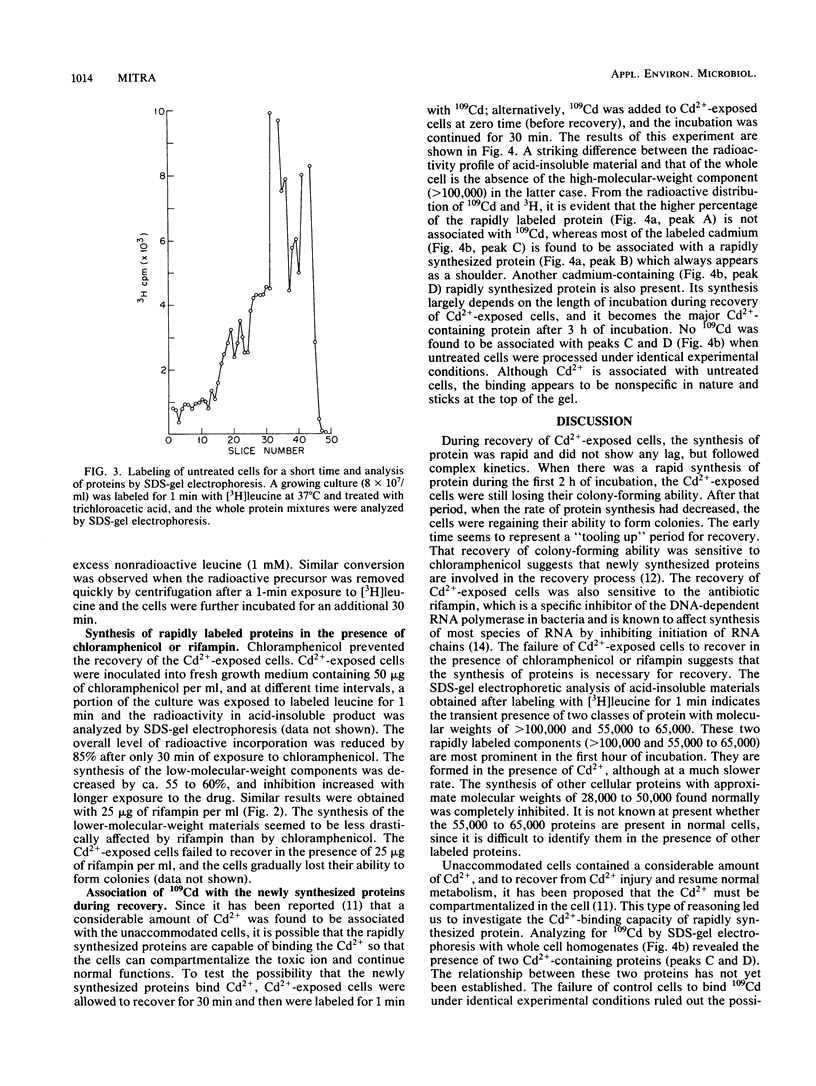
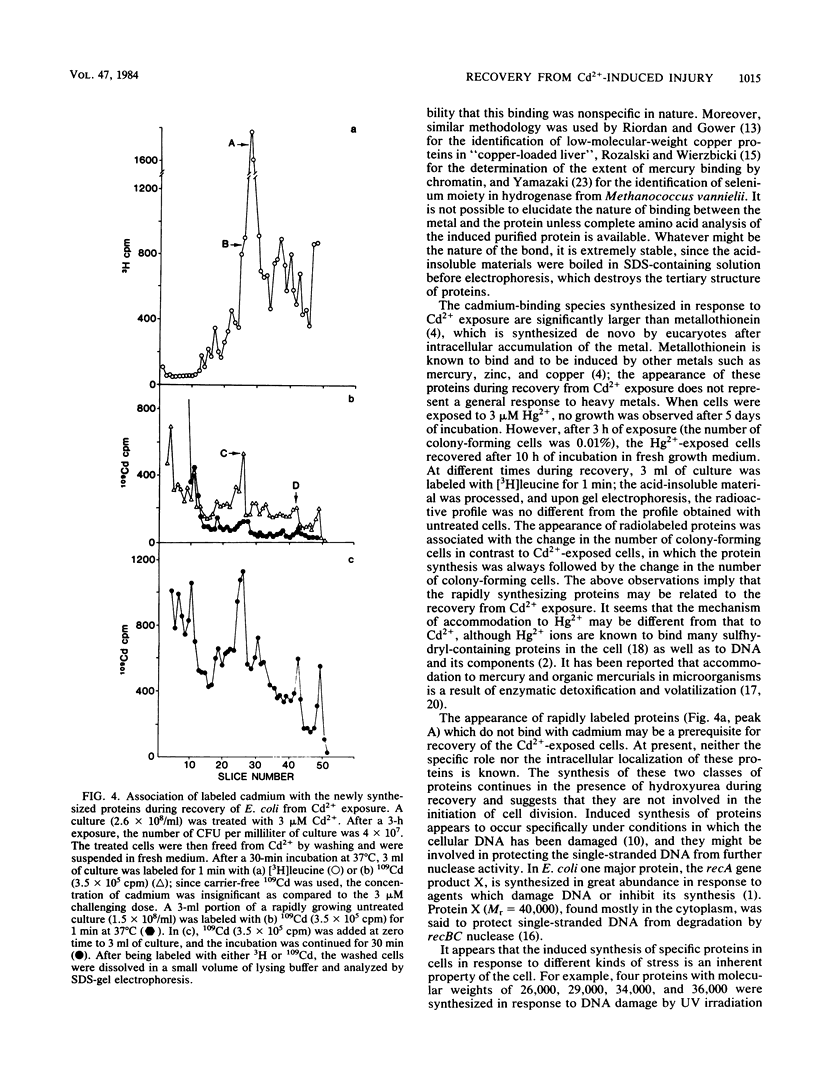

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hansen M. T. Four proteins synthesized in response to deoxyribonucleic acid damage in Micrococcus radiodurans. J Bacteriol. 1980 Jan;141(1):81–86. doi: 10.1128/jb.141.1.81-86.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izatt R. M., Christensen J. J., Rytting J. H. Sites and thermodynamic quantities associated with proton and metal ion interaction with ribonucleic acid, deoxyribonucleic acid, and their constituent bases, nucleosides, and nucleotides. Chem Rev. 1971 Oct;71(5):439–481. doi: 10.1021/cr60273a002. [DOI] [PubMed] [Google Scholar]
- Kenkel T., Trela J. M. Protein turnover in the extreme thermophile Thermus aquaticus. J Bacteriol. 1979 Nov;140(2):543–546. doi: 10.1128/jb.140.2.543-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Mitra R. S., Bernstein I. A. Nature of the repair process associated with the recovery of Escherichia coli after exposure to Cd2+. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1450–1455. doi: 10.1016/0006-291x(77)90604-0. [DOI] [PubMed] [Google Scholar]
- Mitra R. S., Bernstein I. A. Single-strand breakage in DNA of Escherichia coli exposed to Cd2+. J Bacteriol. 1978 Jan;133(1):75–80. doi: 10.1128/jb.133.1.75-80.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R. S., Gray R. H., Chin B., Bernstein I. A. Molecular mechanisms of accommodation in Escherichia coli to toxic levels of Cd2+. J Bacteriol. 1975 Mar;121(3):1180–1188. doi: 10.1128/jb.121.3.1180-1188.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierhaus D., Nierhaus K. H. Identification of the chloramphenicol-binding protein in Escherichia coli ribosomes by partial reconstitution. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2224–2228. doi: 10.1073/pnas.70.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. R., Gower I. Purification of low molecular weight copper proteins from copper loaded liver. Biochem Biophys Res Commun. 1975 Sep 16;66(2):678–686. doi: 10.1016/0006-291x(75)90563-x. [DOI] [PubMed] [Google Scholar]
- Riva S., Silvestri L. G. Rifamycins: a general view. Annu Rev Microbiol. 1972;26:199–224. doi: 10.1146/annurev.mi.26.100172.001215. [DOI] [PubMed] [Google Scholar]
- Rózalski M., Wierzbicki R. Binding of mercury by chromatin of rats exposed to mercuric chloride. Environ Res. 1979 Dec;20(2):465–469. doi: 10.1016/0013-9351(79)90021-5. [DOI] [PubMed] [Google Scholar]
- Satta G., Pardee A. B. Inhibition of Escherichia coli division by protein X. J Bacteriol. 1978 Mar;133(3):1492–1500. doi: 10.1128/jb.133.3.1492-1500.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka T., Tonomura K. Purification and properties of a second enzyme catalyzing the splitting of carbon-mercury linkages from mercury-resistant Pseudomonas K-62. J Bacteriol. 1978 Jul;135(1):138–143. doi: 10.1128/jb.135.1.138-143.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Mallie R. J., Garvey J. S. Radioimmunoassay of metallothioneins. J Biol Chem. 1979 Sep 10;254(17):8416–8421. [PubMed] [Google Scholar]
- Wehrli W., Staehelin M. The rifamycins--relation of chemical structure and action on RNA polymerase. Biochim Biophys Acta. 1969 May 20;182(1):24–29. doi: 10.1016/0005-2787(69)90516-4. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Murphy S. D., Silver S. Mercury and organomercurial resistances determined by plasmids in Staphylococcus aureus. J Bacteriol. 1977 Oct;132(1):197–208. doi: 10.1128/jb.132.1.197-208.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C., Emmerson P. T. Induction of protein synthesis in Escherichia coli following UV- or gamma-irradiation, mitomycin C treatment or tif Expression. Mol Gen Genet. 1977 Feb 28;151(1):57–67. doi: 10.1007/BF00446913. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Ito K., Nakamura Y., Yura T. Transient regulation of protein synthesis in Escherichia coli upon shift-up of growth temperature. J Bacteriol. 1978 Jun;134(3):1133–1140. doi: 10.1128/jb.134.3.1133-1140.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S. A selenium-containing hydrogenase from Methanococcus vannielii. Identification of the selenium moiety as a selenocysteine residue. J Biol Chem. 1982 Jul 25;257(14):7926–7929. [PubMed] [Google Scholar]


