Abstract
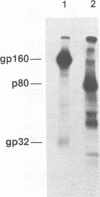
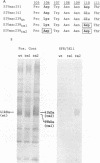
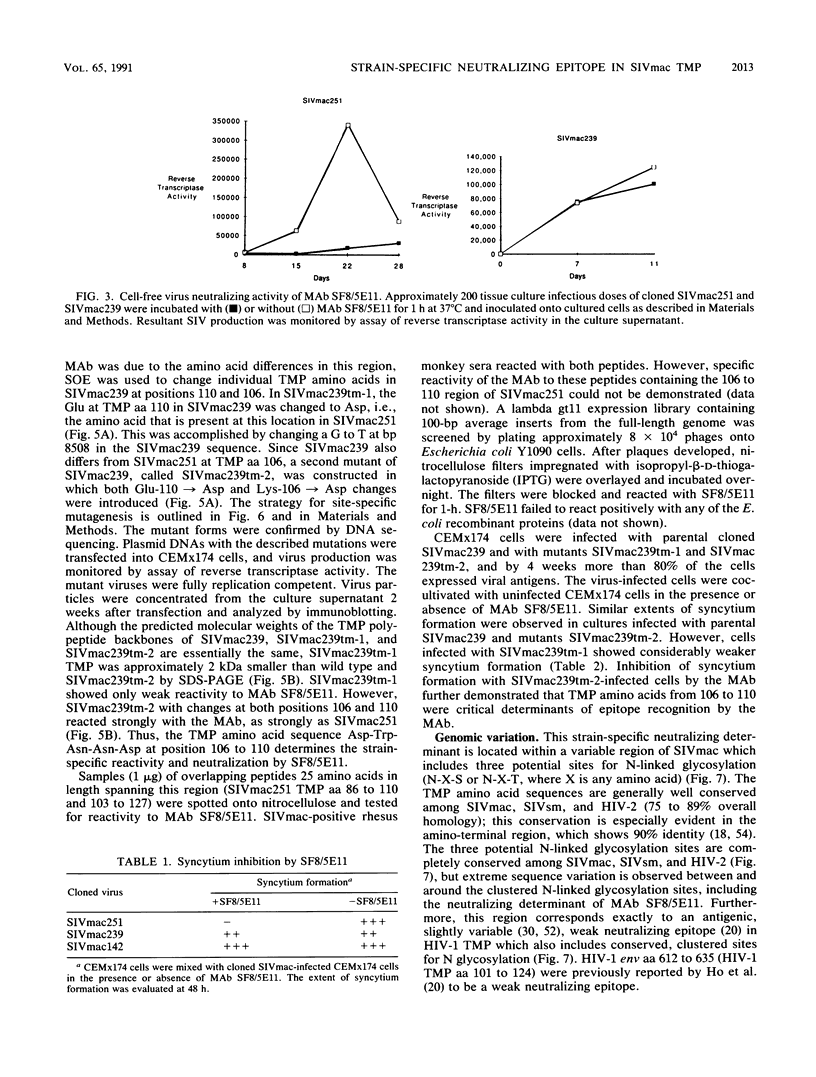
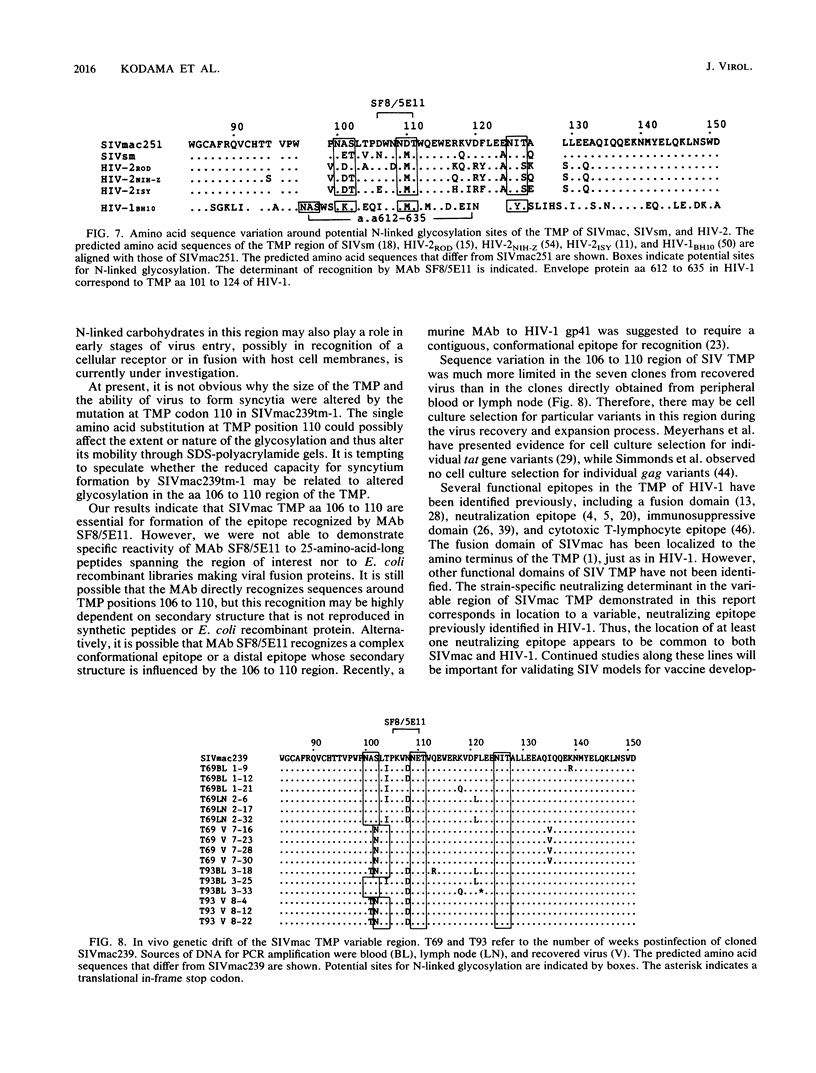
Monoclonal antibody SF8/5E11, which recognizes the transmembrane protein (TMP) of simian immunodeficiency virus of macaque monkeys (SIVmac), displayed strict strain specificity. It reacted with cloned and uncloned SIVmac251 but not with cloned SIVmac142 and SIVmac239 on immunoblots. This monoclonal antibody neutralized infection by cloned, cell-free SIVmac251 and inhibited formation of syncytia by cloned SIVmac251-infected cells; these activities were specific to cloned SIVmac251 and did not occur with the other viruses. Site-specific mutagenesis was used to show that TMP amino acids 106 to 110 (Asp-Trp-Asn-Asn-Asp) determined the strain specificity of the monoclonal antibody. This strain-specific neutralizing determinant is located within a variable region of SIVmac and human immunodeficiency virus type 2 (HIV-2) which includes conserved, clustered sites for N-linked glycosylation. The determinant corresponds exactly to a variable, weak neutralizing epitope in HIV-1 TMP which also includes conserved, clustered sites for N-linked glycosylation. Thus, the location of at least one neutralizing epitope appears to be common to both SIVmac and HIV-1. Our results suggest a role for this determinant in the viral entry process. Genetic variation was observed in this neutralizing determinant following infection of a rhesus monkey with molecularly cloned SIVmac239; variant forms of the strain-specific, neutralizing determinant accumulated during persistent infection in vivo. Selective pressure from the host immune response in vivo may result in sequence variation in this neutralizing determinant.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosch M. L., Earl P. L., Fargnoli K., Picciafuoco S., Giombini F., Wong-Staal F., Franchini G. Identification of the fusion peptide of primate immunodeficiency viruses. Science. 1989 May 12;244(4905):694–697. doi: 10.1126/science.2541505. [DOI] [PubMed] [Google Scholar]
- Burns D. P., Desrosiers R. C. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J Virol. 1991 Apr;65(4):1843–1854. doi: 10.1128/jvi.65.4.1843-1854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Chanh T. C., Dreesman G. R., Kanda P., Linette G. P., Sparrow J. T., Ho D. D., Kennedy R. C. Induction of anti-HIV neutralizing antibodies by synthetic peptides. EMBO J. 1986 Nov;5(11):3065–3071. doi: 10.1002/j.1460-2075.1986.tb04607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Chanh T. C., Kennedy R. C., Kanda P., Clapham P. R., Weiss R. A. Neutralization of diverse HIV-1 strains by monoclonal antibodies raised against a gp41 synthetic peptide. Virology. 1988 Jul;165(1):209–215. doi: 10.1016/0042-6822(88)90674-5. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., Sehgal P. K., Hunsmann G., Schmidt D. K., King N. W., Desrosiers R. C. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987 Dec;68(Pt 12):3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- Desai S. M., Kalyanaraman V. S., Casey J. M., Srinivasan A., Andersen P. R., Devare S. G. Molecular cloning and primary nucleotide sequence analysis of a distinct human immunodeficiency virus isolate reveal significant divergence in its genomic sequences. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8380–8384. doi: 10.1073/pnas.83.21.8380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Daniel M. D., Li Y. HIV-related lentiviruses of nonhuman primates. AIDS Res Hum Retroviruses. 1989 Oct;5(5):465–473. doi: 10.1089/aid.1989.5.465. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Glycosylation inhibitors for N-linked glycoproteins. Methods Enzymol. 1987;138:661–709. doi: 10.1016/0076-6879(87)38060-7. [DOI] [PubMed] [Google Scholar]
- Franchini G., Fargnoli K. A., Giombini F., Jagodzinski L., De Rossi A., Bosch M., Biberfeld G., Fenyo E. M., Albert J., Gallo R. C. Molecular and biological characterization of a replication competent human immunodeficiency type 2 (HIV-2) proviral clone. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2433–2437. doi: 10.1073/pnas.86.7.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz P. N., McClure H. M., Anderson D. C., Swenson R. B., Anand R., Srinivasan A. Isolation of a T-lymphotropic retrovirus from naturally infected sooty mangabey monkeys (Cercocebus atys). Proc Natl Acad Sci U S A. 1986 Jul;83(14):5286–5290. doi: 10.1073/pnas.83.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher W. R. Detection of a fusion peptide sequence in the transmembrane protein of human immunodeficiency virus. Cell. 1987 Jul 31;50(3):327–328. doi: 10.1016/0092-8674(87)90485-5. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader M., Emerman M., Sonigo P., Clavel F., Montagnier L., Alizon M. Genome organization and transactivation of the human immunodeficiency virus type 2. Nature. 1987 Apr 16;326(6114):662–669. doi: 10.1038/326662a0. [DOI] [PubMed] [Google Scholar]
- Hahn B. H., Gonda M. A., Shaw G. M., Popovic M., Hoxie J. A., Gallo R. C., Wong-Staal F. Genomic diversity of the acquired immune deficiency syndrome virus HTLV-III: different viruses exhibit greatest divergence in their envelope genes. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4813–4817. doi: 10.1073/pnas.82.14.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. E., Clausen H., Nielsen C., Teglbjaerg L. S., Hansen L. L., Nielsen C. M., Dabelsteen E., Mathiesen L., Hakomori S. I., Nielsen J. O. Inhibition of human immunodeficiency virus (HIV) infection in vitro by anticarbohydrate monoclonal antibodies: peripheral glycosylation of HIV envelope glycoprotein gp120 may be a target for virus neutralization. J Virol. 1990 Jun;64(6):2833–2840. doi: 10.1128/jvi.64.6.2833-2840.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V. M., Olmsted R. A., Murphey-Corb M., Purcell R. H., Johnson P. R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989 Jun 1;339(6223):389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Kaplan J. C., Rackauskas I. E., Gurney M. E. Second conserved domain of gp120 is important for HIV infectivity and antibody neutralization. Science. 1988 Feb 26;239(4843):1021–1023. doi: 10.1126/science.2830667. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Sarngadharan M. G., Hirsch M. S., Schooley R. T., Rota T. R., Kennedy R. C., Chanh T. C., Sato V. L. Human immunodeficiency virus neutralizing antibodies recognize several conserved domains on the envelope glycoproteins. J Virol. 1987 Jun;61(6):2024–2028. doi: 10.1128/jvi.61.6.2024-2028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
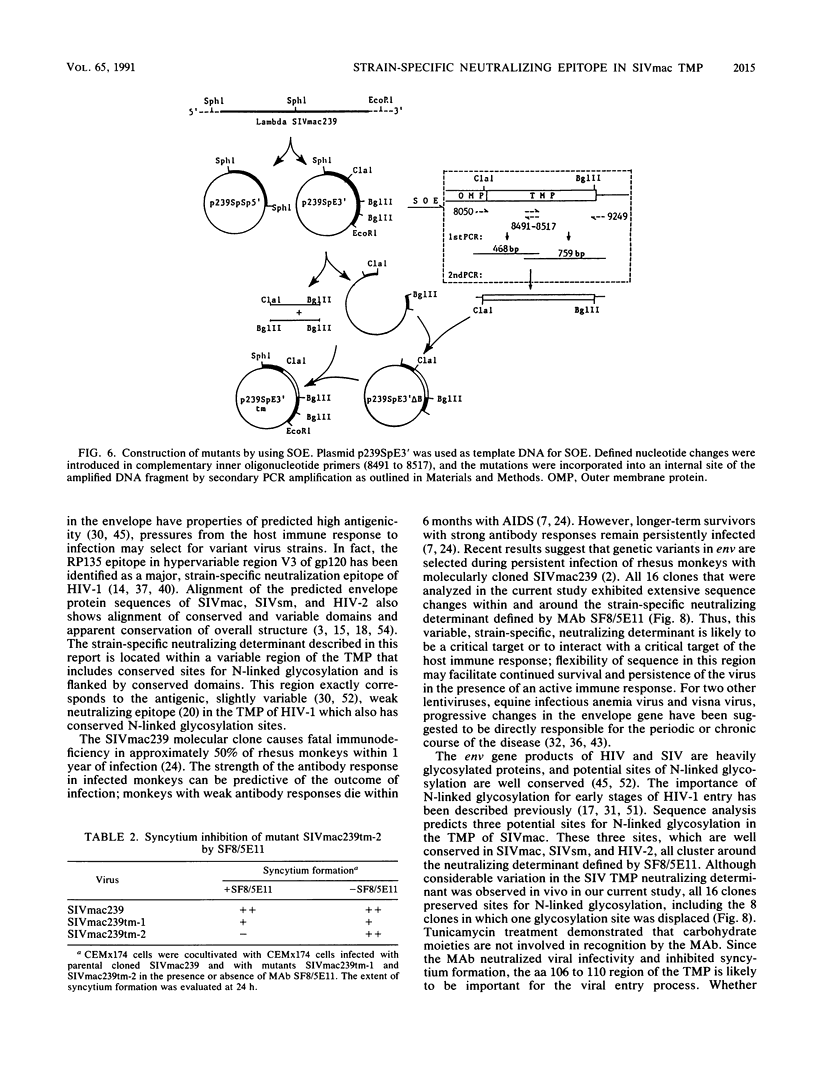
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Hunt J. C., Desai S. M., Casey J. M., Bolling T. J., Leung T. K., Decker R. H., Devare S. G., Sarin V. Mouse monoclonal antibody 5-21-3 recognizes a contiguous, conformation-dependent epitope and maps to a hydrophilic region in HIV-1 gp41. AIDS Res Hum Retroviruses. 1990 May;6(5):587–598. doi: 10.1089/aid.1990.6.587. [DOI] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Li Y., Naidu Y. M., Butler C. V., Ochs M. F., Jaenel G., King N. W., Daniel M. D., Desrosiers R. C. Comparison of simian immunodeficiency virus isolates. Nature. 1988 Feb 18;331(6157):619–622. doi: 10.1038/331619a0. [DOI] [PubMed] [Google Scholar]
- Kestler H., Kodama T., Ringler D., Marthas M., Pedersen N., Lackner A., Regier D., Sehgal P., Daniel M., King N. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990 Jun 1;248(4959):1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- Klasse P. J., Pipkorn R., Blomberg J. Presence of antibodies to a putatively immunosuppressive part of human immunodeficiency virus (HIV) envelope glycoprotein gp41 is strongly associated with health among HIV-positive subjects. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5225–5229. doi: 10.1073/pnas.85.14.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Wooley D. P., Naidu Y. M., Kestler H. W., 3rd, Daniel M. D., Li Y., Desrosiers R. C. Significance of premature stop codons in env of simian immunodeficiency virus. J Virol. 1989 Nov;63(11):4709–4714. doi: 10.1128/jvi.63.11.4709-4714.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori D. C., Robinson W. E., Jr, Mitchell W. M. Role of protein N-glycosylation in pathogenesis of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9248–9252. doi: 10.1073/pnas.85.23.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Parekh B., Orrego A., Issel C. J. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J Biol Chem. 1984 Aug 25;259(16):10539–10544. [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Rangan S. R., Baskin G. B., Gormus B. J., Wolf R. H., Andes W. A., West M., Montelaro R. C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986 May 22;321(6068):435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- Naidu Y. M., Kestler H. W., 3rd, Li Y., Butler C. V., Silva D. P., Schmidt D. K., Troup C. D., Sehgal P. K., Sonigo P., Daniel M. D. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988 Dec;62(12):4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Masuda T., Tsujimoto H., Ishikawa K., Kodama T., Morikawa S., Nakai M., Honjo S., Hayami M. Isolation of simian immunodeficiency virus from African green monkeys and seroepidemiologic survey of the virus in various non-human primates. Int J Cancer. 1988 Jan 15;41(1):115–122. doi: 10.1002/ijc.2910410121. [DOI] [PubMed] [Google Scholar]
- Payne S. L., Salinovich O., Nauman S. M., Issel C. J., Montelaro R. C. Course and extent of variation of equine infectious anemia virus during parallel persistent infections. J Virol. 1987 Apr;61(4):1266–1270. doi: 10.1128/jvi.61.4.1266-1270.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Regier D. A., Desrosiers R. C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- Ruegg C. L., Monell C. R., Strand M. Inhibition of lymphoproliferation by a synthetic peptide with sequence identity to gp41 of human immunodeficiency virus type 1. J Virol. 1989 Aug;63(8):3257–3260. doi: 10.1128/jvi.63.8.3257-3260.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. V., Stowring L., Haase A. T., Narayan O., Vigne R. Antigenic variation in visna virus. Cell. 1979 Oct;18(2):321–327. doi: 10.1016/0092-8674(79)90051-5. [DOI] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Peutherer J. F., Ludlam C. A., Bishop J. O., Brown A. J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990 Feb;64(2):864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcich B. R., Hahn B. H., Shaw G. M., McNeely P. D., Modrow S., Wolf H., Parks E. S., Parks W. P., Josephs S. F., Gallo R. C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986 Jun 6;45(5):637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Cohen J., Hosmalin A., Cease K. B., Houghten R., Cornette J. L., DeLisi C., Moss B., Germain R. N., Berzofsky J. A. An immunodominant epitope of the human immunodeficiency virus envelope glycoprotein gp160 recognized by class I major histocompatibility complex molecule-restricted murine cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3105–3109. doi: 10.1073/pnas.85.9.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto H., Cooper R. W., Kodama T., Fukasawa M., Miura T., Ohta Y., Ishikawa K., Nakai M., Frost E., Roelants G. E. Isolation and characterization of simian immunodeficiency virus from mandrills in Africa and its relationship to other human and simian immunodeficiency viruses. J Virol. 1988 Nov;62(11):4044–4050. doi: 10.1128/jvi.62.11.4044-4050.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese F. D., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Biochemical and immunological analysis of human immunodeficiency virus gag gene products p17 and p24. J Virol. 1988 Mar;62(3):795–801. doi: 10.1128/jvi.62.3.795-801.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese F. D., Joseph B., Copeland T. D., Oroszlan S., Gallo R. C., Sarngadharan M. G. Identification of simian immunodeficiency virus SIVMAC env gene products. J Virol. 1989 Mar;63(3):1416–1419. doi: 10.1128/jvi.63.3.1416-1419.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Kowalski M., Goh W. C., Kozarsky K., Krieger M., Rosen C., Rohrschneider L., Haseltine W. A., Sodroski J. Inhibition of human immunodeficiency virus syncytium formation and virus replication by castanospermine. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8120–8124. doi: 10.1073/pnas.84.22.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Rutledge R. A., Dias S., Folks T., Theodore T., Buckler C. E., Martin M. A. Identification of conserved and divergent domains within the envelope gene of the acquired immunodeficiency syndrome retrovirus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5038–5042. doi: 10.1073/pnas.83.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Shaw G. M., Hahn B. H., Salahuddin S. Z., Popovic M., Markham P., Redfield R., Gallo R. C. Genomic diversity of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Aug 23;229(4715):759–762. doi: 10.1126/science.2992084. [DOI] [PubMed] [Google Scholar]
- Zagury J. F., Franchini G., Reitz M., Collalti E., Starcich B., Hall L., Fargnoli K., Jagodzinski L., Guo H. G., Laure F. Genetic variability between isolates of human immunodeficiency virus (HIV) type 2 is comparable to the variability among HIV type 1. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5941–5945. doi: 10.1073/pnas.85.16.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]