Abstract
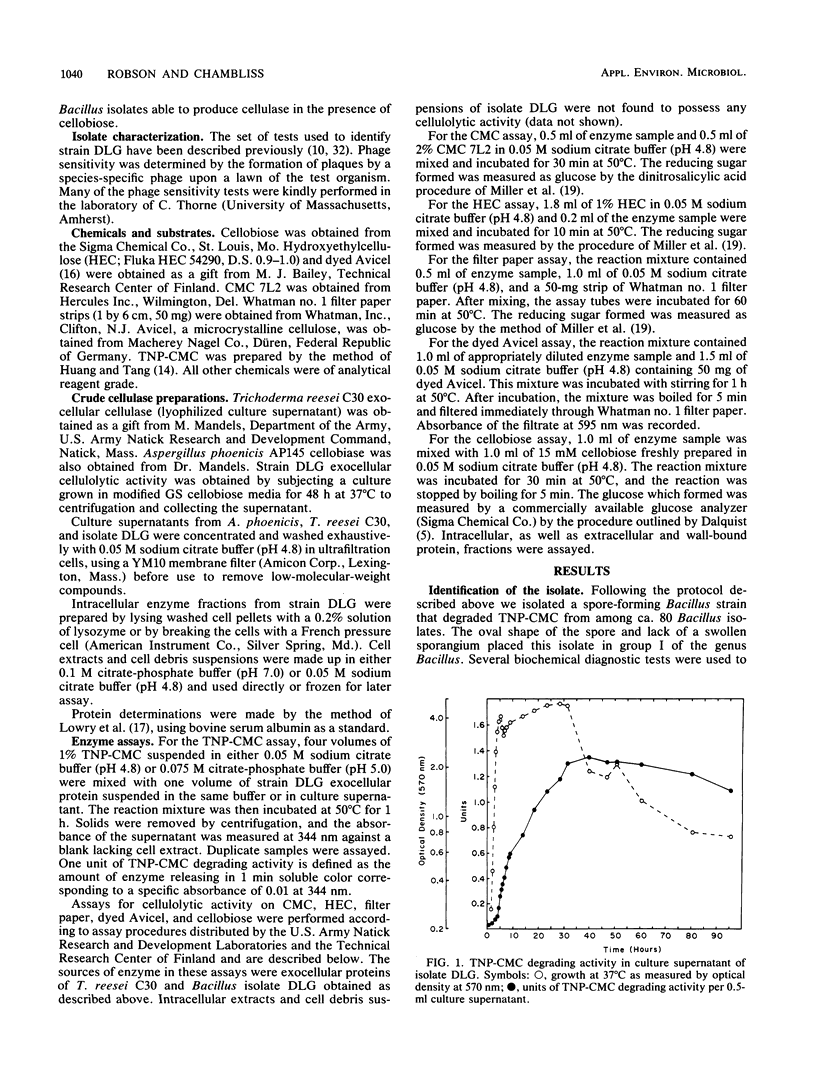
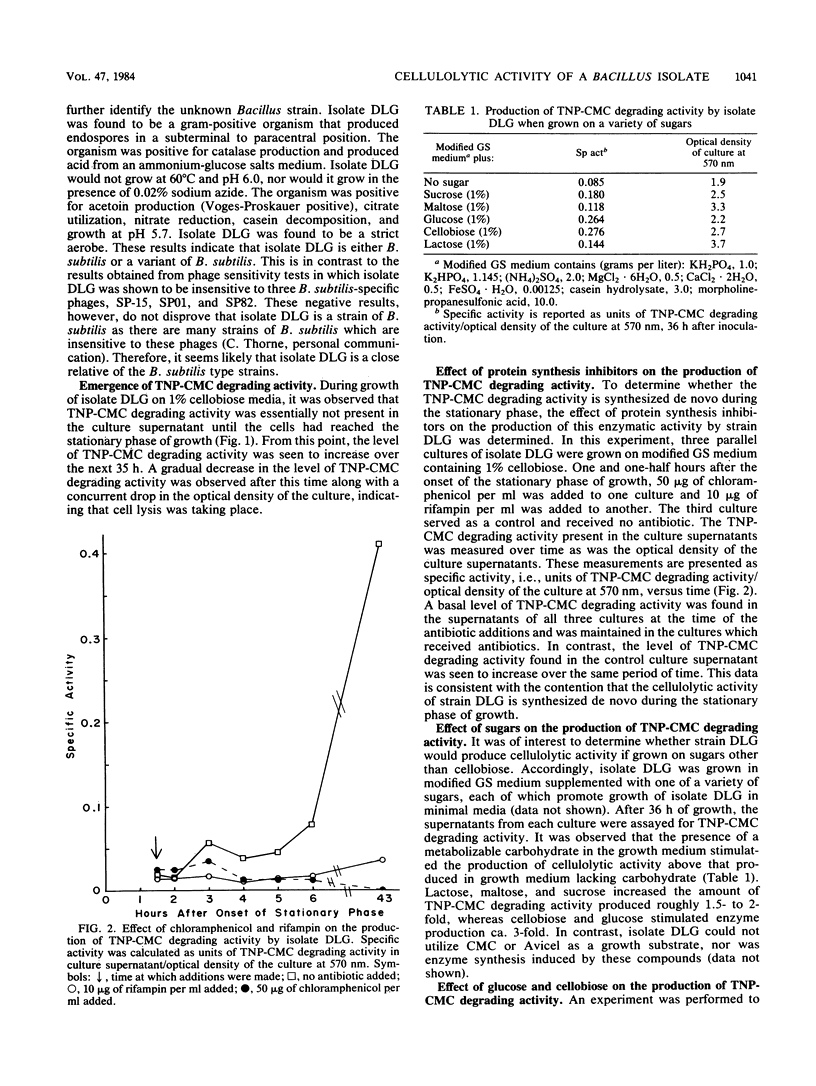
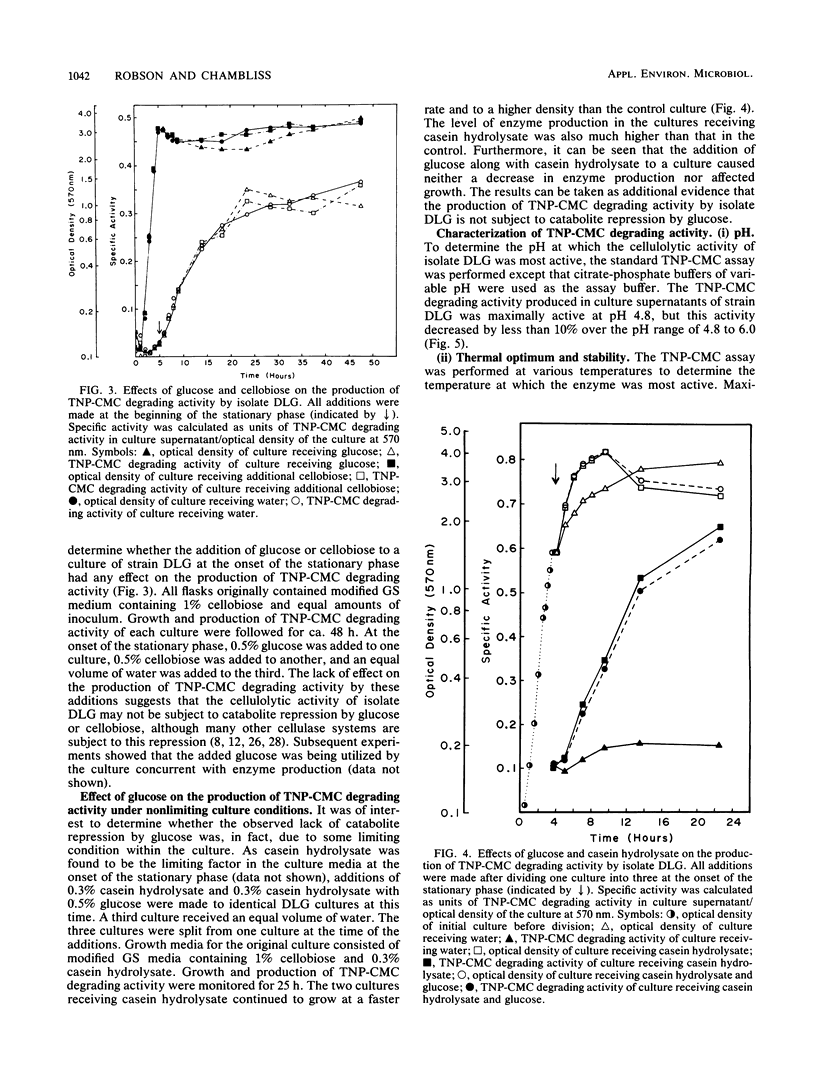
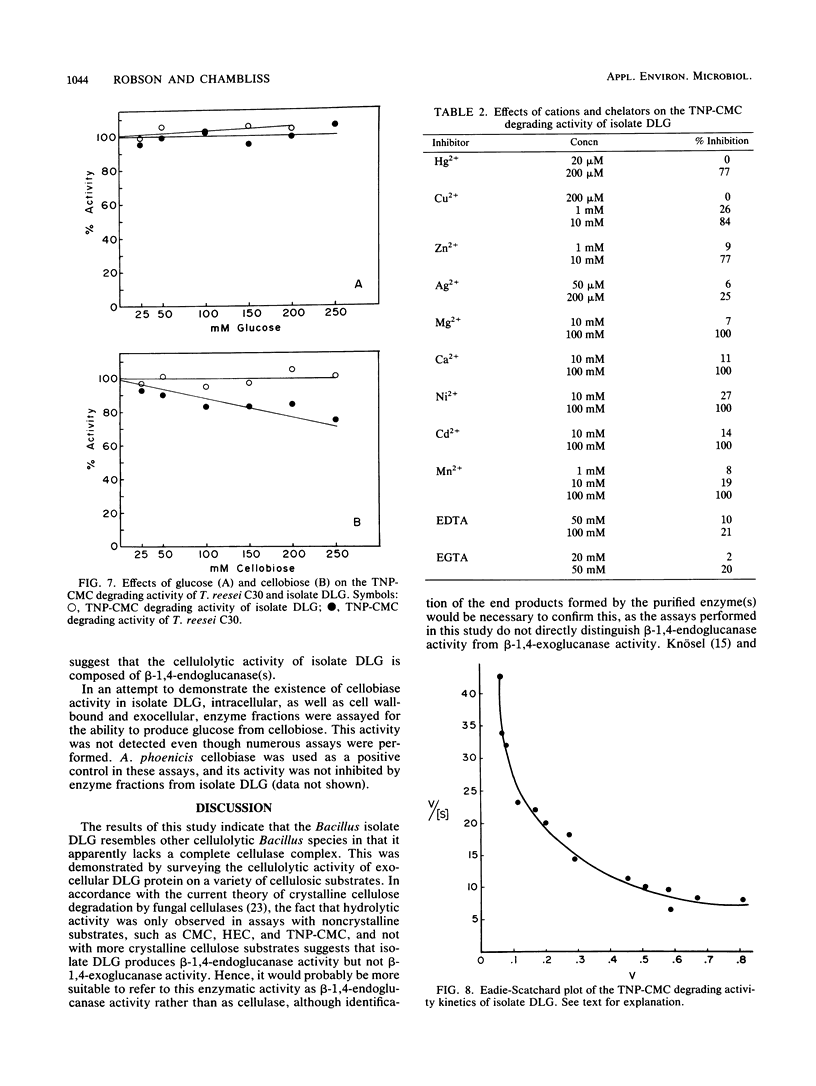
A group I Bacillus strain, DLG, was isolated and characterized as being most closely related to Bacillus subtilis. When grown on any of a variety of sugars, the culture supernatant of this isolate was found to possess cellulolytic activity, as demonstrated by degradation of trinitrophenyl-carboxymethyl cellulose. Growth in medium containing cellobiose or glucose resulted in the greatest production of cellulolytic activity. The cellulolytic activity was not produced until the stationary phase of growth, and the addition of glucose or cellobiose to a culture in this phase had no apparent effect on enzyme production. Fractionation of the culture supernatant showed that the molecular weight of the enzymatic activity was less than 100,000. Maximum cellulolytic activity in assays was observed at pH 4.8 and at 58C, although maximum thermal stability of the activity. Kinetic experiments suggested that more than one enzyme was acting upon trinitrophenyl-carboxymethyl cellulose. Exocellular protein produced by this Bacillus isolate showed roughly one-fifth the cellulolytic activity displayed by Trichoderma reesei C30 on noncrystalline, cellulosic substrates. In contrast to T. reesei cellulase, the Bacillus enzymatic activity showed no ability to degrade crystalline forms of cellulose, nor was cellobiase activity detectable.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER J. K. Characteristics of cellobiose phosphorylase. J Bacteriol. 1961 Jun;81:903–910. doi: 10.1128/jb.81.6.903-910.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AYERS W. A. Phosphorylation of cellobiose and glucose by Ruminococcus flavefaciens. J Bacteriol. 1958 Nov;76(5):515–517. doi: 10.1128/jb.76.5.515-517.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghem L. E., Pettersson L. G., Axiö-Fredriksson U. B. The mechanism of enzymatic cellulose degradation. Purification and some properties of two different 1,4beta-glucan glucanohydrolases from Trichoderma viride. Eur J Biochem. 1976 Jan 15;61(2):621–630. doi: 10.1111/j.1432-1033.1976.tb10058.x. [DOI] [PubMed] [Google Scholar]
- Bissett F. H. Analysis of cellulase proteins by high-performance liquid chromatography. J Chromatogr. 1979 Oct 31;178(2):515–523. doi: 10.1016/s0021-9673(00)92510-x. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. Determination of maltase and isomaltase activities with a glucose-oxidase reagent. Biochem J. 1961 Sep;80:547–551. doi: 10.1042/bj0800547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusee M. C., Leatherwood J. M. Regulation of cellulase from Ruminococcus. Can J Microbiol. 1972 Mar;18(3):347–353. doi: 10.1139/m72-053. [DOI] [PubMed] [Google Scholar]
- Herr D. Secretion of cellulase and beta-glucosidase by Trichoderma viride ITCC-1433 in submerged culture on different substrates. Biotechnol Bioeng. 1979 Aug;21(8):1361–1371. doi: 10.1002/bit.260210805. [DOI] [PubMed] [Google Scholar]
- Huang J. S., Tang J. Sensitive assay for cellulase and dextranase. Anal Biochem. 1976 Jun;73(2):369–377. doi: 10.1016/0003-2697(76)90182-2. [DOI] [PubMed] [Google Scholar]
- Hägerdal B. G., Ferchak J. D., Pye E. K. Cellulolytic Enzyme System of Thermoactinomyces sp. Grown on Microcrystalline Cellulose. Appl Environ Microbiol. 1978 Oct;36(4):606–612. doi: 10.1128/aem.36.4.606-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knösel D. Fortgeführte Untersuchungen zur pektolytischen und cellulolytischen Aktivität verschiedener Bacillus-Species. Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1971;126(6):604–609. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ng T. K., Zeikus J. G. Comparison of Extracellular Cellulase Activities of Clostridium thermocellum LQRI and Trichoderma reesei QM9414. Appl Environ Microbiol. 1981 Aug;42(2):231–240. doi: 10.1128/aem.42.2.231-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Zeikus J. G. Purification and characterization of an endoglucanase (1,4-beta-D-glucan glucanohydrolase) from Clostridium thermocellum. Biochem J. 1981 Nov 1;199(2):341–350. doi: 10.1042/bj1990341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddler J. N., Khan A. W. Cellulolytic enzyme system of Acetivibrio cellulolyticus. Can J Microbiol. 1981 Mar;27(3):288–294. doi: 10.1139/m81-045. [DOI] [PubMed] [Google Scholar]
- Saddler J. N., Khan A. W. Cellulose degradation by a new isolate from sewage sludge, a member of the Bacteroidaceae family. Can J Microbiol. 1979 Dec;25(12):1427–1432. doi: 10.1139/m79-222. [DOI] [PubMed] [Google Scholar]
- Saddler J. N., Khan A. W., Martin S. M. Regulation of cellulase synthesis in Acetivibrio cellulolyticus. Microbios. 1980;28(112):97–106. [PubMed] [Google Scholar]
- Shoemaker S. P., Brown R. D., Jr Characterization of endo-1,4-beta-D-glucanases purified from Trichoderma viride. Biochim Biophys Acta. 1978 Mar 14;523(1):147–161. doi: 10.1016/0005-2744(78)90017-7. [DOI] [PubMed] [Google Scholar]
- Stoppok W., Rapp P., Wagner F. Formation, Location, and Regulation of Endo-1,4-beta-Glucanases and beta-Glucosidases from Cellulomonas uda. Appl Environ Microbiol. 1982 Jul;44(1):44–53. doi: 10.1128/aem.44.1.44-53.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer D. W. Carboxymethylcellulase produced by facultative bacteria from the hind-gut of the termite Reticulitermes hesperus. J Gen Microbiol. 1978 May;106(1):13–18. doi: 10.1099/00221287-106-1-13. [DOI] [PubMed] [Google Scholar]
- Umezurike G. M. The cellulolytic enzymes of Botryodiplodia theobromae Pat. Separation and characterization of cellulases and beta-glucosidases. Biochem J. 1979 Jan 1;177(1):9–19. doi: 10.1042/bj1770009. [DOI] [PMC free article] [PubMed] [Google Scholar]


