Abstract
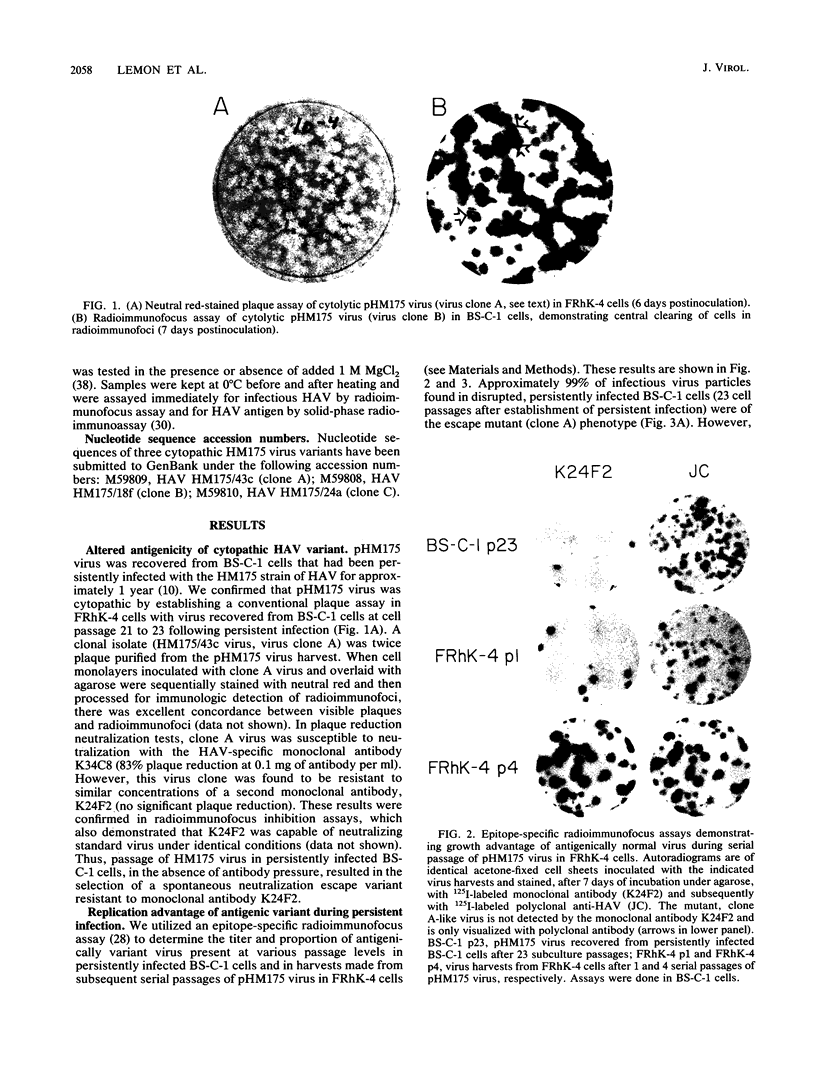
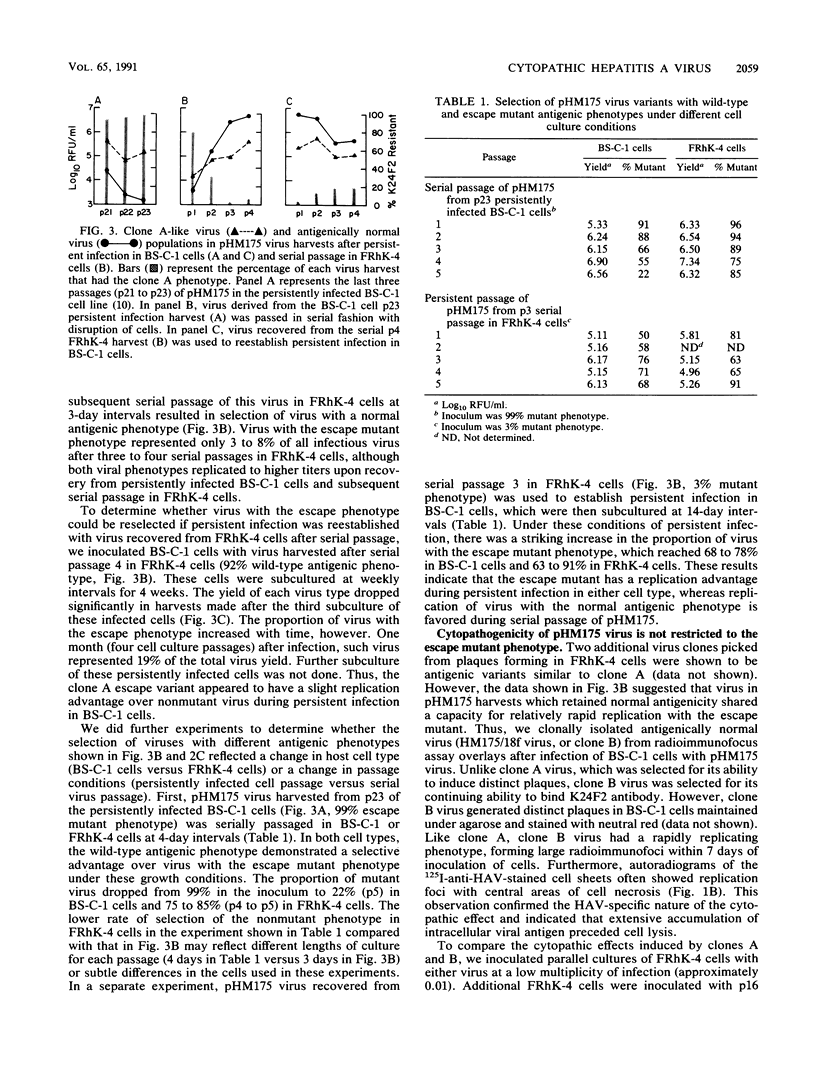
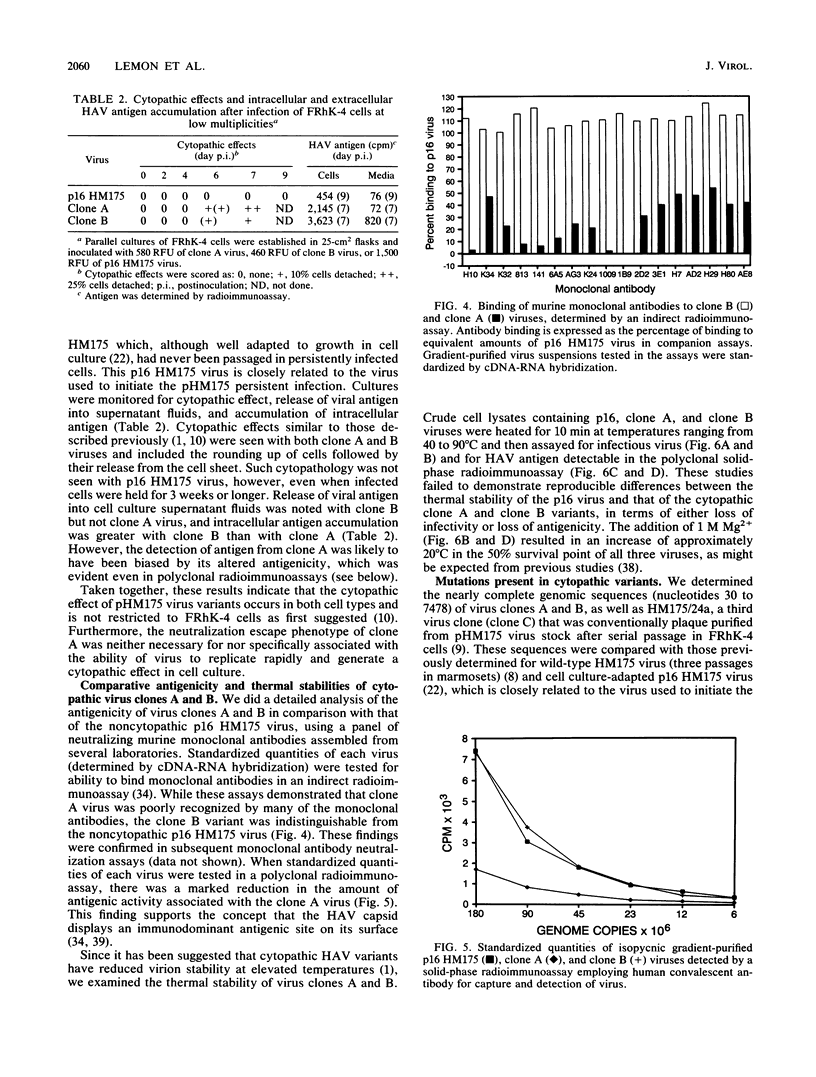
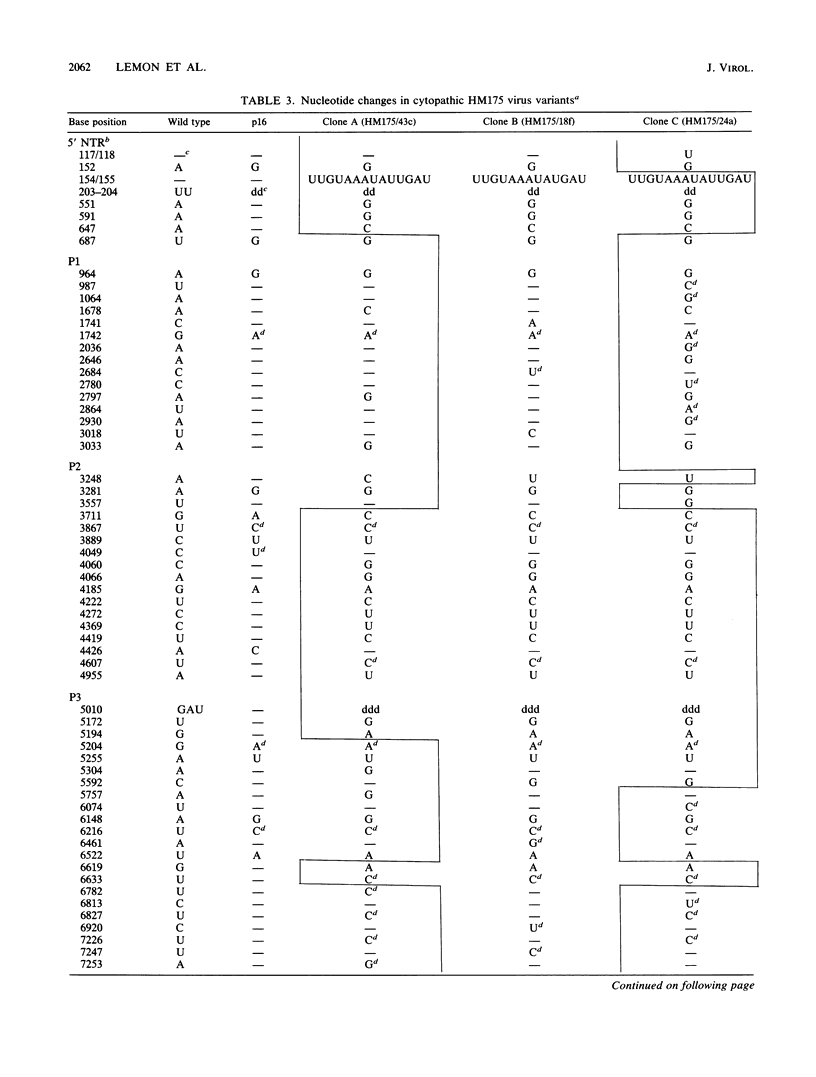
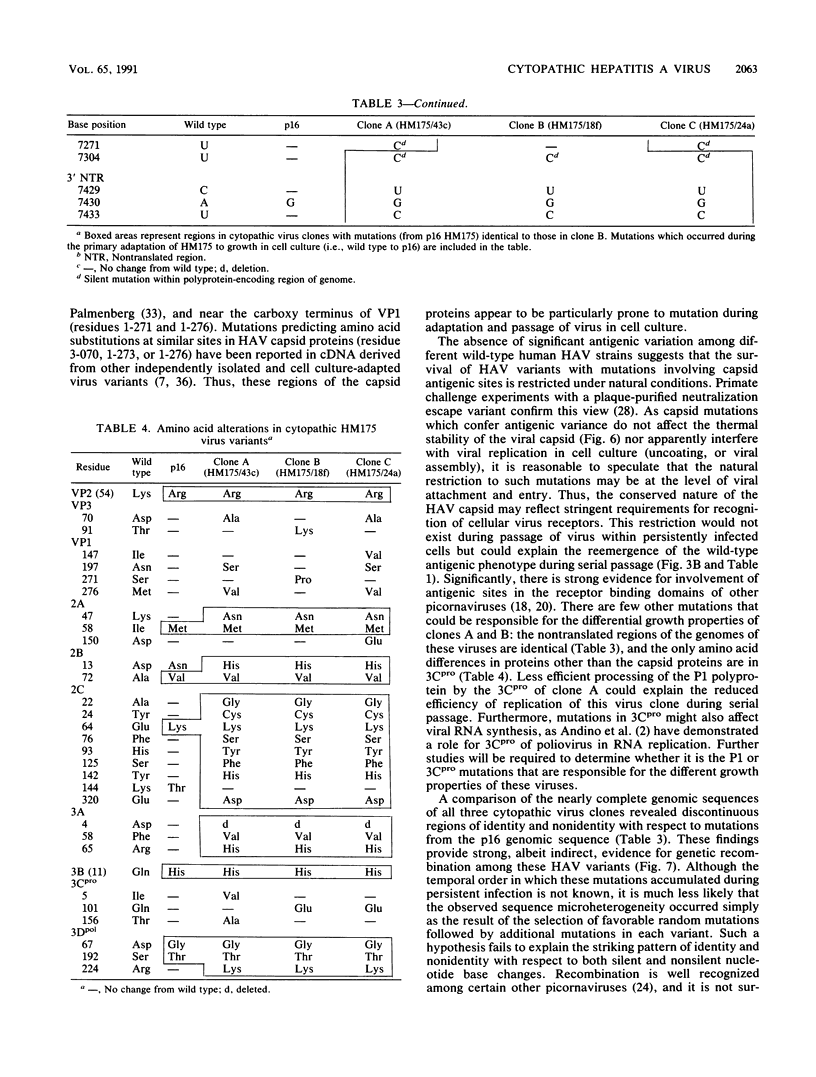
Variants of hepatitis A virus (pHM175 virus) recovered from persistently infected green monkey kidney (BS-C-1) cells induced a cytopathic effect during serial passage in BS-C-1 or fetal rhesus kidney (FRhK-4) cells. Epitope-specific radioimmunofocus assays showed that this virus comprised two virion populations, one with altered antigenicity including neutralization resistance to monoclonal antibody K24F2, and the other with normal antigenic characteristics. Replication of the antigenic variant was favored over that of virus with the normal antigenic phenotype during persistent infection, while virus with the normal antigenic phenotype was selected during serial passage. Viruses of each type were clonally isolated; both were cytopathic in cell cultures and displayed a rapid replication phenotype when compared with the noncytopathic passage 16 (p16) HM175 virus which was used to establish the original persistent infection. The two cytopathic virus clones contained 31 and 34 nucleotide changes from the sequence of p16 HM175. Both shared a common 5' sequence (bases 30 to 1677), as well as sequence identity in the P2-P3 region (bases 3249 to 5303 and 6462 to 6781) and 3' terminus (bases 7272 to 7478). VP3, VP1, and 3Cpro contained different mutations in the two virus clones, with amino acid substitutions at residues 70 of VP3 and 197 and 276 of VP1 of the antigenic variant. These capsid mutations did not affect virion thermal stability. A comparison of the nearly complete genomic sequences of three clonally isolated cytopathic variants was suggestive of genetic recombination between these viruses during persistent infection and indicated that mutations in both 5' and 3' nontranslated regions and in the nonstructural proteins 2A, 2B, 2C, 3A, and 3Dpol may be related to the cytopathic phenotype.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. A. Cytopathology, plaque assay, and heat inactivation of hepatitis A virus strain HM175. J Med Virol. 1987 May;22(1):35–44. doi: 10.1002/jmv.1890220106. [DOI] [PubMed] [Google Scholar]
- Andino R., Rieckhof G. E., Trono D., Baltimore D. Substitutions in the protease (3Cpro) gene of poliovirus can suppress a mutation in the 5' noncoding region. J Virol. 1990 Feb;64(2):607–612. doi: 10.1128/jvi.64.2.607-612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz K., Egger D., Troxler M., Pasamontes L. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J Virol. 1990 Mar;64(3):1156–1163. doi: 10.1128/jvi.64.3.1156-1163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binn L. N., Lemon S. M., Marchwicki R. H., Redfield R. R., Gates N. L., Bancroft W. H. Primary isolation and serial passage of hepatitis A virus strains in primate cell cultures. J Clin Microbiol. 1984 Jul;20(1):28–33. doi: 10.1128/jcm.20.1.28-33.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Rosenblum B., Feinstone S. M., Ticehurst J., Purcell R. H. Attenuation and cell culture adaptation of hepatitis A virus (HAV): a genetic analysis with HAV cDNA. J Virol. 1989 Dec;63(12):5364–5370. doi: 10.1128/jvi.63.12.5364-5370.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Rosenblum B., Ticehurst J. R., Daemer R. J., Feinstone S. M., Purcell R. H. Complete nucleotide sequence of an attenuated hepatitis A virus: comparison with wild-type virus. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2497–2501. doi: 10.1073/pnas.84.8.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromeans T., Fields H. A., Sobsey M. D. Replication kinetics and cytopathic effect of hepatitis A virus. J Gen Virol. 1989 Aug;70(Pt 8):2051–2062. doi: 10.1099/0022-1317-70-8-2051. [DOI] [PubMed] [Google Scholar]
- Cromeans T., Sobsey M. D., Fields H. A. Development of a plaque assay for a cytopathic, rapidly replicating isolate of hepatitis A virus. J Med Virol. 1987 May;22(1):45–56. doi: 10.1002/jmv.1890220107. [DOI] [PubMed] [Google Scholar]
- Daemer R. J., Feinstone S. M., Gust I. D., Purcell R. H. Propagation of human hepatitis A virus in African green monkey kidney cell culture: primary isolation and serial passage. Infect Immun. 1981 Apr;32(1):388–393. doi: 10.1128/iai.32.1.388-393.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G. J., Decker R. H., Norton D. K., Bryce W. H., Whittington R. O., Tribby I. I., Mushahwar I. K. Monoclonal antibodies to hepatitis A virus. J Med Virol. 1984;14(1):1–8. doi: 10.1002/jmv.1890140102. [DOI] [PubMed] [Google Scholar]
- De Chastonay J., Siegl G. Replicative events in hepatitis A virus-infected MRC-5 cells. Virology. 1987 Apr;157(2):268–275. doi: 10.1016/0042-6822(87)90269-8. [DOI] [PubMed] [Google Scholar]
- Flehmig B. Hepatitis A-virus in cell culture: I. propagation of different hepatitis A-virus isolates in a fetal rhesus monkey kidney cell line (Frhk-4). Med Microbiol Immunol. 1980;168(4):239–248. doi: 10.1007/BF02121807. [DOI] [PubMed] [Google Scholar]
- Fox G., Parry N. R., Barnett P. V., McGinn B., Rowlands D. J., Brown F. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J Gen Virol. 1989 Mar;70(Pt 3):625–637. doi: 10.1099/0022-1317-70-3-625. [DOI] [PubMed] [Google Scholar]
- Gauss-Müller V., Deinhardt F. Effect of hepatitis A virus infection on cell metabolism in vitro. Proc Soc Exp Biol Med. 1984 Jan;175(1):10–15. doi: 10.3181/00379727-175-41757. [DOI] [PubMed] [Google Scholar]
- Gregersen J. P., Mehdi S., Mauler R. Adaptation of hepatitis A virus to high titre growth in diploid and permanent cell cultures. Med Microbiol Immunol. 1988;177(2):91–100. doi: 10.1007/BF00189530. [DOI] [PubMed] [Google Scholar]
- Jansen R. W., Newbold J. E., Lemon S. M. Complete nucleotide sequence of a cell culture-adapted variant of hepatitis A virus: comparison with wild-type virus with restricted capacity for in vitro replication. Virology. 1988 Apr;163(2):299–307. doi: 10.1016/0042-6822(88)90270-x. [DOI] [PubMed] [Google Scholar]
- Jansen R. W., Siegl G., Lemon S. M. Molecular epidemiology of human hepatitis A virus defined by an antigen-capture polymerase chain reaction method. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2867–2871. doi: 10.1073/pnas.87.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N. Antigenic relatedness of two strains of hepatitis A virus determined by cross-neutralization. Infect Immun. 1983 Oct;42(1):418–420. doi: 10.1128/iai.42.1.418-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N., Marchwicki R. H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983 May;17(5):834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N., Marchwicki R., Murphy P. C., Ping L. H., Jansen R. W., Asher L. V., Stapleton J. T., Taylor D. G., LeDuc J. W. In vivo replication and reversion to wild type of a neutralization-resistant antigenic variant of hepatitis A virus. J Infect Dis. 1990 Jan;161(1):7–13. doi: 10.1093/infdis/161.1.7. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Jansen R. W. A simple method for clonal selection of hepatitis A virus based on recovery of virus from radioimmunofocus overlays. J Virol Methods. 1985 Jun;11(2):171–176. doi: 10.1016/0166-0934(85)90040-0. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., LeDuc J. W., Binn L. N., Escajadillo A., Ishak K. G. Transmission of hepatitis A virus among recently captured Panamanian owl monkeys. J Med Virol. 1982;10(1):25–36. doi: 10.1002/jmv.1890100105. [DOI] [PubMed] [Google Scholar]
- Lemon S. M. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985 Oct 24;313(17):1059–1067. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- MacGregor A., Kornitschuk M., Hurrell J. G., Lehmann N. I., Coulepis A. G., Locarnini S. A., Gust I. D. Monoclonal antibodies against hepatitis A virus. J Clin Microbiol. 1983 Nov;18(5):1237–1243. doi: 10.1128/jcm.18.5.1237-1243.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasser A. M., Metcalf T. G. Production of cytopathology in FRhK-4 cells by BS-C-1-passaged hepatitis A virus. Appl Environ Microbiol. 1987 Dec;53(12):2967–2971. doi: 10.1128/aem.53.12.2967-2971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping L. H., Jansen R. W., Stapleton J. T., Cohen J. I., Lemon S. M. Identification of an immunodominant antigenic site involving the capsid protein VP3 of hepatitis A virus. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8281–8285. doi: 10.1073/pnas.85.21.8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost P. J., Hilleman M. R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979 Feb;160(2):213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]
- Ross B. C., Anderson B. N., Edwards P. C., Gust I. D. Nucleotide sequence of high-passage hepatitis A virus strain HM175: comparison with wild-type and cell culture-adapted strains. J Gen Virol. 1989 Oct;70(Pt 10):2805–2810. doi: 10.1099/0022-1317-70-10-2805. [DOI] [PubMed] [Google Scholar]
- Siegl G., Weitz M., Kronauer G. Stability of hepatitis A virus. Intervirology. 1984;22(4):218–226. doi: 10.1159/000149554. [DOI] [PubMed] [Google Scholar]
- Stapleton J. T., Lemon S. M. Neutralization escape mutants define a dominant immunogenic neutralization site on hepatitis A virus. J Virol. 1987 Feb;61(2):491–498. doi: 10.1128/jvi.61.2.491-498.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ticehurst J. R., Racaniello V. R., Baroudy B. M., Baltimore D., Purcell R. H., Feinstone S. M. Molecular cloning and characterization of hepatitis A virus cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5885–5889. doi: 10.1073/pnas.80.19.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbracht A., Hofmann L., Wurster K. G., Flehmig B. Persistent infection of human fibroblasts by hepatitis A virus. J Gen Virol. 1984 Mar;65(Pt 3):609–615. doi: 10.1099/0022-1317-65-3-609. [DOI] [PubMed] [Google Scholar]
- Venuti A., Di Russo C., del Grosso N., Patti A. M., Ruggeri F., De Stasio P. R., Martiniello M. G., Pagnotti P., Degener A. M., Midulla M. Isolation and molecular cloning of a fast-growing strain of human hepatitis A virus from its double-stranded replicative form. J Virol. 1985 Nov;56(2):579–588. doi: 10.1128/jvi.56.2.579-588.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F. H., Lomax N. B. Host range mutants of human rhinovirus in which nonstructural proteins are altered. J Virol. 1983 Nov;48(2):410–418. doi: 10.1128/jvi.48.2.410-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C., Wimmer E., Holland J. J. Very high frequency of reversion to guanidine resistance in clonal pools of guanidine-dependent type 1 poliovirus. J Virol. 1990 Feb;64(2):664–671. doi: 10.1128/jvi.64.2.664-671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]