Abstract
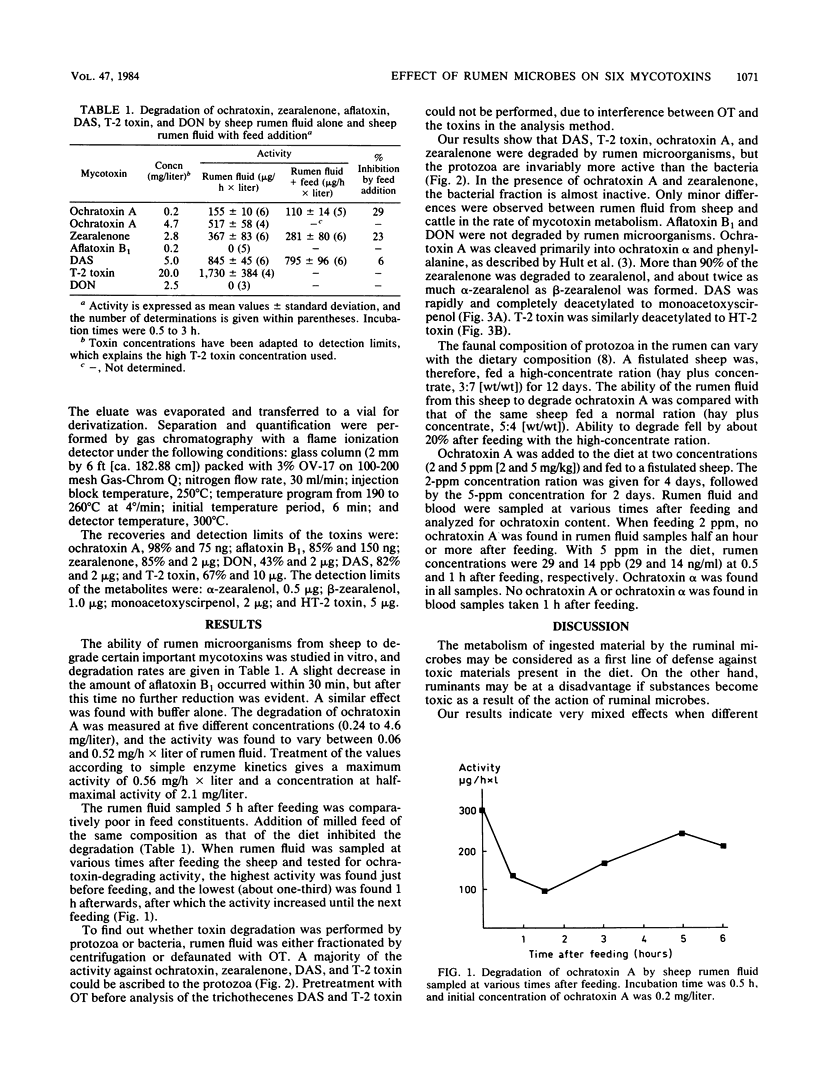
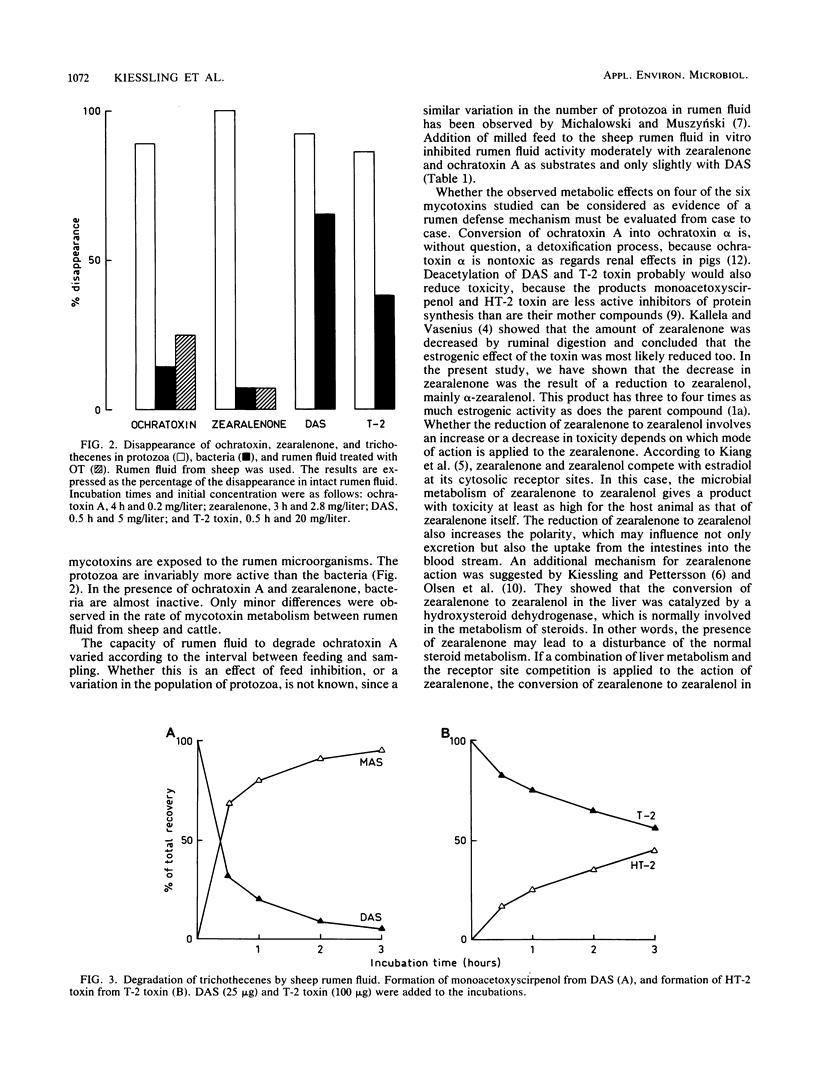
The effect of rumen microbes on six mycotoxins (aflatoxin B1, ochratoxin A, zearalenone, T-2 toxin, diacetoxyscirpenol, and deoxynivalenol ) considered to be health risks for domestic animals was investigated. The mycotoxins were incubated with intact rumen fluid or fractions of rumen protozoa and bacteria from sheep and cattle in the presence or absence of milled feed. Rumen fluid had no effect on aflatoxin B1 and deoxynivalenol . The remaining four mycotoxins were all metabolized, and protozoa were more active than bacteria. Metabolism of ochratoxin A, zearalenone, and diacetoxyscirpenol was moderately or slightly inhibited by addition of milled feed in vitro. The capacity of rumen fluid to degrade ochratoxin A decreased after feeding, but this activity was gradually restored by the next feeding time. Ochratoxin A was cleaved to ochratoxin alpha and phenylalanine; zearalenone was reduced to alpha-zearalenol and to a lesser degree to beta-zearalenol; diacetoxyscirpenol and T-2 toxin were deacetylated to monoacetoxyscirpenol and HT-2 toxin, respectively. Feeding of 5 ppm (5 mg/kg) of ochratoxin A to sheep revealed 14 ppb (14 ng/ml) of ochratoxin A and ochratoxin alpha in rumen fluid after 1 h, but neither was detected in the blood. Whether such conversions in the rumen fluid may be considered as a first line of defense against toxic compounds present in the diet is briefly discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hagler W. M., Mirocha C. J., Pathre S. V., Behrens J. C. Identification of the naturally occurring isomer of zearalenol produced by Fusarium roseum 'Gibbosum' in rice culture. Appl Environ Microbiol. 1979 May;37(5):849–853. doi: 10.1128/aem.37.5.849-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hult K., Gatenbeck S. A spectrophotometric procedure, using carboxypeptidase A, for the quantitative measurement of ochratoxin A. J Assoc Off Anal Chem. 1976 Jan;59(1):128–129. [PubMed] [Google Scholar]
- Hult K., Teiling A., Gatenbeck S. Degradation of ochratoxin A by a ruminant. Appl Environ Microbiol. 1976 Sep;32(3):443–444. doi: 10.1128/aem.32.3.443-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallela K., Vasenius L. The effects of rumen fluid on the content of zearalenone in animal fodder. Nord Vet Med. 1982 Oct;34(10):336–339. [PubMed] [Google Scholar]
- Kiang D. T., Kennedy B. J., Pathre S. V., Mirocha C. J. Binding characteristics of zearalenone analogs to estrogen receptors. Cancer Res. 1978 Nov;38(11 Pt 1):3611–3615. [PubMed] [Google Scholar]
- Kiessling K. H., Pettersson H. Metabolism of zearalenone in rat liver. Acta Pharmacol Toxicol (Copenh) 1978 Oct;43(4):285–290. doi: 10.1111/j.1600-0773.1978.tb02267.x. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Kanegasaki S. Densities of ruminal protozoa of sheep established under different dietary conditions. J Dairy Sci. 1969 Feb;52(2):250–255. doi: 10.3168/jds.S0022-0302(69)86538-0. [DOI] [PubMed] [Google Scholar]
- Ohta M., Matsumoto H., Ishii K., Ueno Y. Metabolism of trichothecene mycotoxins. II. Substrate specificity of microsomal deacetylation of trichothecenes. J Biochem. 1978 Sep;84(3):697–706. doi: 10.1093/oxfordjournals.jbchem.a132175. [DOI] [PubMed] [Google Scholar]
- Olsen M., Pettersson H., Kiessling K. H. Reduction of zearalenone to zearalenol in female rat liver by 3 alpha-hydroxysteroid dehydrogenase. Acta Pharmacol Toxicol (Copenh) 1981 Feb;48(2):157–161. doi: 10.1111/j.1600-0773.1981.tb01602.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki M., Suzuki S., Sakakibara Y., Miyaki K. The toxicity of 5-chloro-8-hydroxy-3, 4-dihydro-3-methyl-isocoumarin-7-carboxylic acid, a hydrolyzate of ochratoxin A. Jpn J Med Sci Biol. 1971 Aug;24(4):245–250. doi: 10.7883/yoken1952.24.245. [DOI] [PubMed] [Google Scholar]


