Abstract
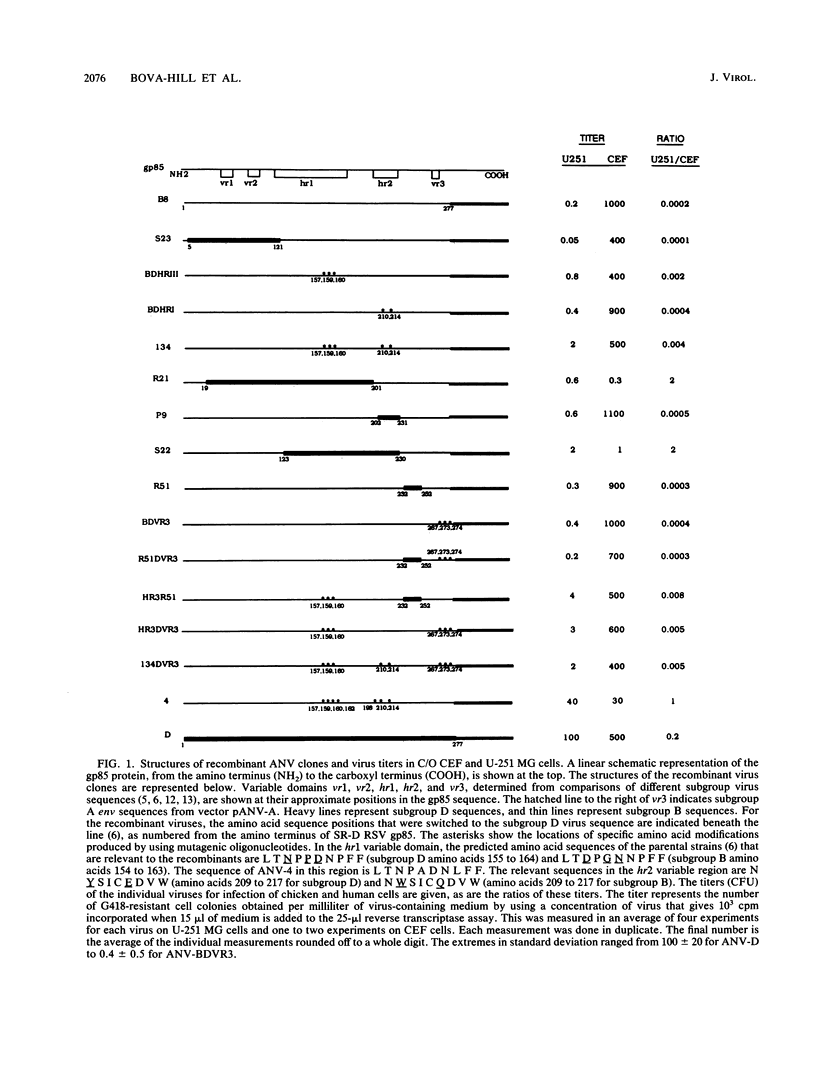
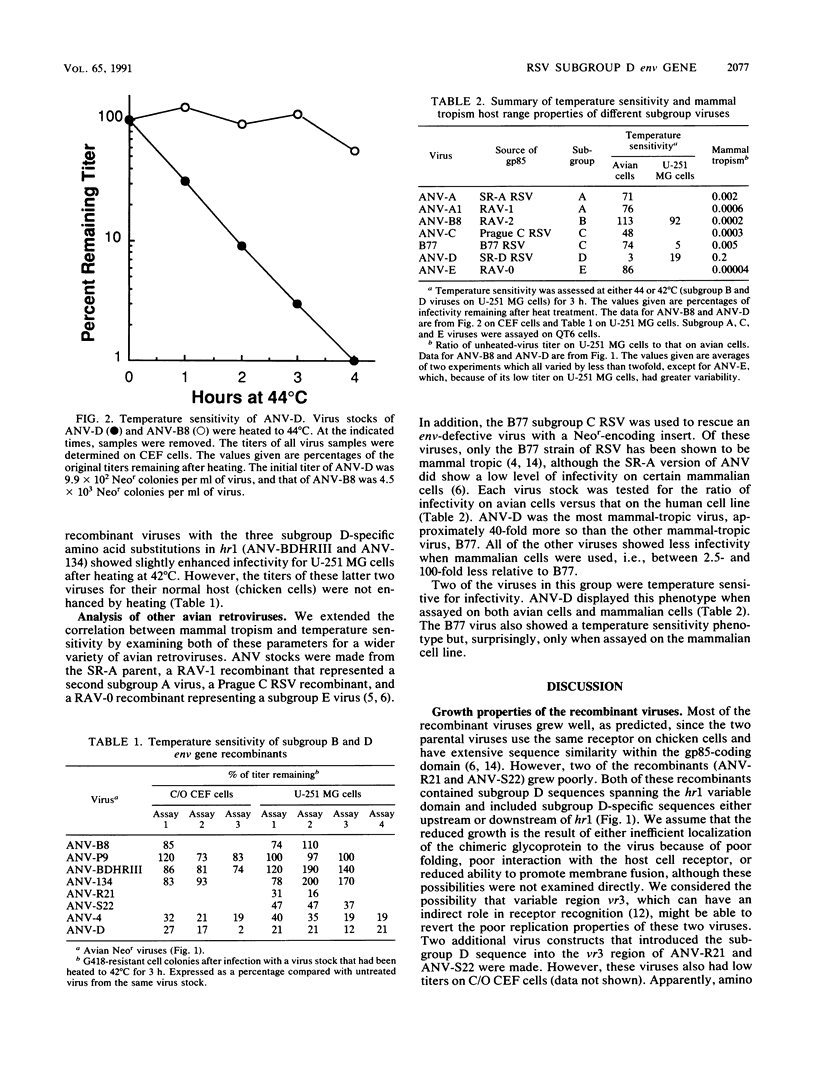
Subgroup D avian sarcoma and leukosis viruses can penetrate a variety of mammalian cells in addition to cells from their natural host, chickens. Sequences derived from the gp85-coding domain within the env gene of a mammal-tropic subgroup D virus (Schmidt-Ruppin D strain of Rous sarcoma virus [SR-D RSV]) and a non-mammal-tropic subgroup B virus (Rous-associated virus type 2) were recombined to map genetic determinants that allow penetration of mammalian cells. The following conclusions were based on host range analysis of the recombinant viruses. (i) The determinants of gp85 that result in the mammal tropism phenotype of SR-D RSV are encoded within the 160 codons that lie 3' of codon 121 from the corresponding amino terminus of the gp85 protein. (ii) Small linear domains of the SR-D RSV gp85-coding domain placed in the subgroup B background did not yield viruses with titers equal to that of the subgroup D virus in a human cell line. (iii) Recombinant viruses that contained subgroup D sequences within the hr1 variable domain of gp85 showed modest-to-significant increases in infectivity on human cells relative to chicken cells. A recombinant virus that contained three fortuitous amino acid substitutions in the gp85-coding domain was found to penetrate the human cell line and give a titer similar to that of the subgroup D virus. In addition, we found that the subgroup D virus, the mutant virus, and recombinant viruses with an increased mammal tropism phenotype were unstable at 42 degrees C. These results suggest that the mammal tropism of the SR-D strain is not related to altered receptor specificity but rather to an unstable and fusogenic viral glycoprotein. A temperature sensitivity phenotype for infectivity of mammalian cells was also observed for another mammal-tropic avian retrovirus, the Bratislava 77 strain of RSV, a subgroup C virus, but was not seen for any other avian retrovirus tested, strengthening the correlation between mammal tropism and temperature sensitivity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer H., Graf T. Evidence for the possible existence of two envelope antigenic determinants and corresponding cell receptors for avian tumor viruses. Virology. 1969 Jan;37(1):157–161. doi: 10.1016/0042-6822(69)90322-5. [DOI] [PubMed] [Google Scholar]
- Bigner D. D., Bigner S. H., Pontén J., Westermark B., Mahaley M. S., Ruoslahti E., Herschman H., Eng L. F., Wikstrand C. J. Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981 May;40(3):201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Love D. N., Weiss R. A. Virus envelope markers in mammalian tropism of avian RNA tumor viruses. J Virol. 1975 Jan;15(1):108–114. doi: 10.1128/jvi.15.1.108-114.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova C. A., Manfredi J. P., Swanstrom R. env genes of avian retroviruses: nucleotide sequence and molecular recombinants define host range determinants. Virology. 1986 Jul 30;152(2):343–354. doi: 10.1016/0042-6822(86)90137-6. [DOI] [PubMed] [Google Scholar]
- Bova C. A., Olsen J. C., Swanstrom R. The avian retrovirus env gene family: molecular analysis of host range and antigenic variants. J Virol. 1988 Jan;62(1):75–83. doi: 10.1128/jvi.62.1.75-83.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRITTENDEN L. B., OKAZAKI W., REAMER R. GENETIC RESISTANCE TO ROUS SARCOMA VIRUS IN EMBRYO CELL CULTURES AND EMBRYOS. Virology. 1963 Jul;20:541–544. doi: 10.1016/0042-6822(63)90107-7. [DOI] [PubMed] [Google Scholar]
- Cao Y. Z., Friedman-Kien A. E., Huang Y. X., Li X. L., Mirabile M., Moudgil T., Zucker-Franklin D., Ho D. D. CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J Virol. 1990 Jun;64(6):2553–2559. doi: 10.1128/jvi.64.6.2553-2559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P. R., Weber J. N., Whitby D., McIntosh K., Dalgleish A. G., Maddon P. J., Deen K. C., Sweet R. W., Weiss R. A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- Crittenden L. B., Stone H. A., Reamer R. H., Okazaki W. Two loci controlling genetic cellular resistance to avian leukosis-sarcoma viruses. J Virol. 1967 Oct;1(5):898–904. doi: 10.1128/jvi.1.5.898-904.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Stoye J. P., Coffin J. M. Molecular basis of host range variation in avian retroviruses. J Virol. 1985 Jan;53(1):32–39. doi: 10.1128/jvi.53.1.32-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- Gilbert J. M., Mason D., White J. M. Fusion of Rous sarcoma virus with host cells does not require exposure to low pH. J Virol. 1990 Oct;64(10):5106–5113. doi: 10.1128/jvi.64.10.5106-5113.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harouse J. M., Kunsch C., Hartle H. T., Laughlin M. A., Hoxie J. A., Wigdahl B., Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S., Kosik E. Mutagenesis of the region between env and src of the SR-A strain of Rous sarcoma virus for the purpose of constructing helper-independent vectors. Virology. 1984 Jul 15;136(1):89–99. doi: 10.1016/0042-6822(84)90250-2. [DOI] [PubMed] [Google Scholar]
- Hunter E., Hill E., Hardwick M., Bhown A., Schwartz D. E., Tizard R. Complete sequence of the Rous sarcoma virus env gene: identification of structural and functional regions of its product. J Virol. 1983 Jun;46(3):920–936. doi: 10.1128/jvi.46.3.920-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter E., Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- Joyce C. M., Grindley N. D. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol. 1984 May;158(2):636–643. doi: 10.1128/jb.158.2.636-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M. New procedure for DNA transfection with polycation and dimethyl sulfoxide. Mol Cell Biol. 1984 Jun;4(6):1172–1174. doi: 10.1128/mcb.4.6.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kunsch C., Hartle H. T., Wigdahl B. Infection of human fetal dorsal root ganglion glial cells with human immunodeficiency virus type 1 involves an entry mechanism independent of the CD4 T4A epitope. J Virol. 1989 Dec;63(12):5054–5061. doi: 10.1128/jvi.63.12.5054-5061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. L., Moudgil T., Vinters H. V., Ho D. D. CD4-independent, productive infection of a neuronal cell line by human immunodeficiency virus type 1. J Virol. 1990 Mar;64(3):1383–1387. doi: 10.1128/jvi.64.3.1383-1387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Olsen J. C., Bova-Hill C., Grandgenett D. P., Quinn T. P., Manfredi J. P., Swanstrom R. Rearrangements in unintegrated retroviral DNA are complex and are the result of multiple genetic determinants. J Virol. 1990 Nov;64(11):5475–5484. doi: 10.1128/jvi.64.11.5475-5484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE L. N., BIGGS P. M. DIFFERENCES BETWEEN HIGHLY INBRED LINES OF CHICKENS IN THE RESPONSE TO ROUS SARCOMA VIRUS OF THE CHORIOALLANTOIC MEMBRANE AND OF EMBRYONIC CELLS IN TISSUE CULTURE. Virology. 1964 Dec;24:610–616. doi: 10.1016/0042-6822(64)90215-6. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Ruigrok R. W., Martin S. R., Wharton S. A., Skehel J. J., Bayley P. M., Wiley D. C. Conformational changes in the hemagglutinin of influenza virus which accompany heat-induced fusion of virus with liposomes. Virology. 1986 Dec;155(2):484–497. doi: 10.1016/0042-6822(86)90210-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Zelenetz A. D., Cooper G. M. Molecular cloning and characterization of the chicken gene homologous to the transforming gene of Rous sarcoma virus. Cell. 1981 May;24(2):531–541. doi: 10.1016/0092-8674(81)90344-5. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Steimer K. S., Boettiger D. Complementation rescue of Rous sarcoma virus from transformed mammalian cells by polyethylene glycol-mediated cell fusion. J Virol. 1977 Jul;23(1):133–141. doi: 10.1128/jvi.23.1.133-141.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereba A., Murti K. G. A very sensitive biochemical assay for detecting and quantitating avian oncornaviruses. Virology. 1977 Jul 1;80(1):166–176. doi: 10.1016/0042-6822(77)90389-0. [DOI] [PubMed] [Google Scholar]
- Toyoshima K., Vogt P. K. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology. 1969 Jul;38(3):414–426. doi: 10.1016/0042-6822(69)90154-8. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Quintrell N., Wyke J. Revertants of an ASV-transformed rat cell line have lost the complete provius or sustained mutations in src. Virology. 1981 Jan 15;108(1):28–46. doi: 10.1016/0042-6822(81)90525-0. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Envelope classification of avian RNA tumor viruses. Bibl Haematol. 1970;(36):153–167. doi: 10.1159/000391704. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Patterns of viral interference in the avian leukosis and sarcoma complex. Virology. 1966 Nov;30(3):368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]



