Abstract
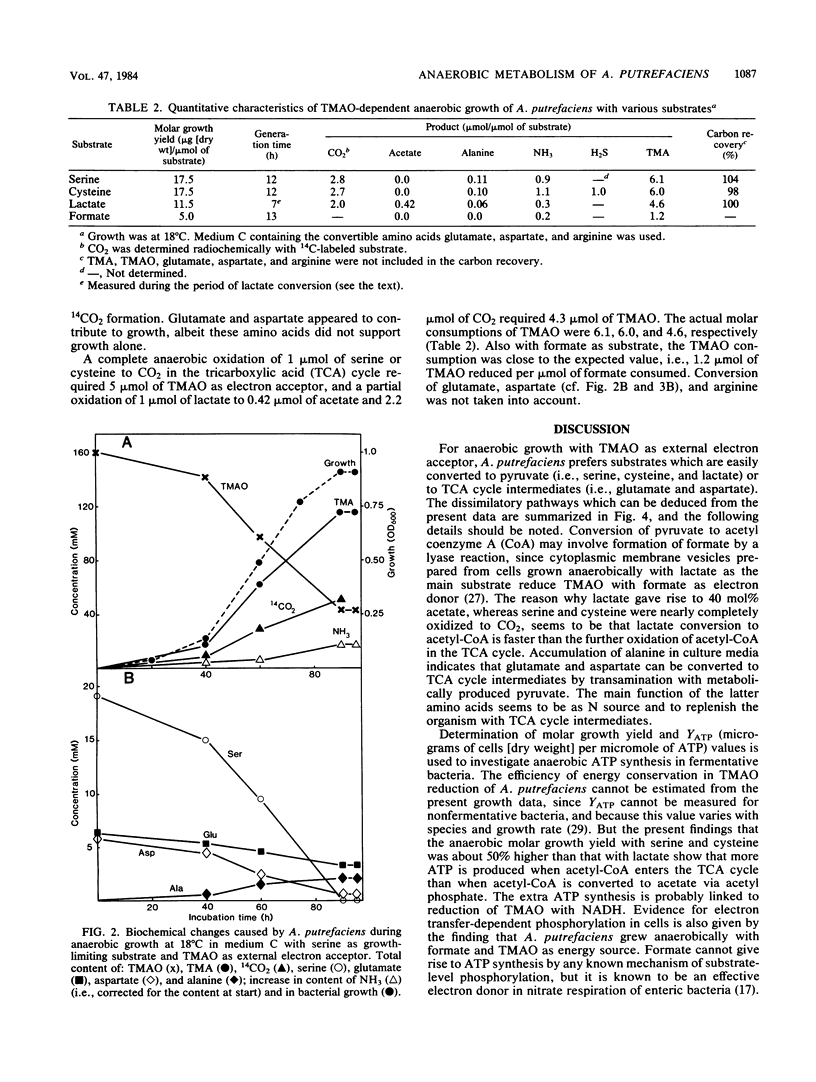
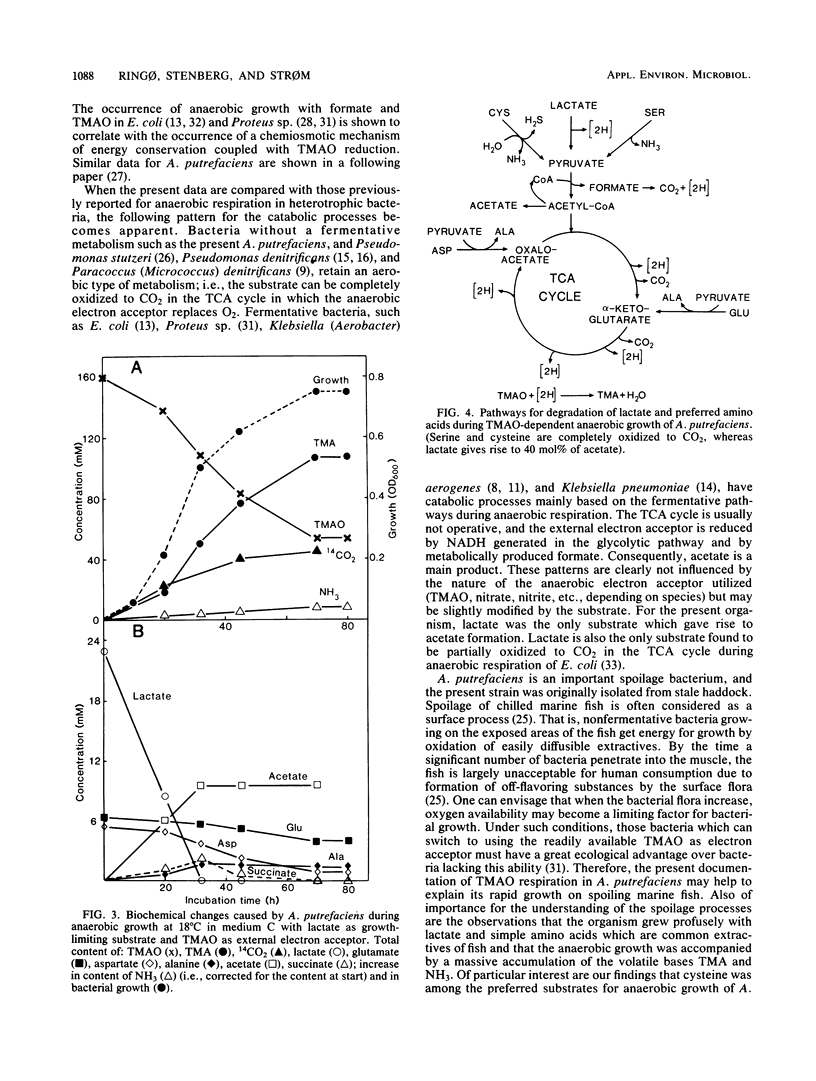
The nonfermentative Alteromonas putrefaciens NCMB 1735 grew anaerobically in defined media with trimethylamine oxide as external electron acceptor. All amino acids tested, except taurine and those with a cyclic or aromatic side chain, were utilized during trimethylamine oxide-dependent anaerobic growth. Lactate, serine, and cysteine (which are easily converted to pyruvate) and glutamate and aspartate (which are easily converted to tricarboxylic acid cycle intermediates) were metabolized at the fastest rate. Growth with lactate as growth-limiting substrate gave rise to the formation of 40 mol% acetate, whereas serine and cysteine were nearly completely oxidized to CO2. Molar growth yields with the latter substrates were the same and were 50% higher than with lactate. This showed that more ATP was formed when acetyl coenzyme A entered the tricarboxylic acid cycle than when it was converted via acetyl phosphate to acetate. Also, growth with formate as substrate indicated that the reduction of trimethylamine oxide to trimethylamine was coupled with energy conservation by a respiratory mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agústsson I., Strøm A. R. Biosynthesis and turnover of trimethylamine oxide in the teleost cod, Gadus morhua. J Biol Chem. 1981 Aug 10;256(15):8045–8049. [PubMed] [Google Scholar]
- Brendel K., Meezan E. A simple apparatus for the continuous monitoring of 14CO2 production from several small reaction mixtures. Anal Biochem. 1974 Jul;60(1):88–101. doi: 10.1016/0003-2697(74)90133-x. [DOI] [PubMed] [Google Scholar]
- Conway E. J., Byrne A. An absorption apparatus for the micro-determination of certain volatile substances: The micro-determination of ammonia. Biochem J. 1933;27(2):419–429. [PMC free article] [PubMed] [Google Scholar]
- Cox J. C., Madigan M. T., Favinger J. L., Gest H. Redox mechanisms in "oxidant-dependent" hexose fermentation by Rhodopseudomonas capsulata. Arch Biochem Biophys. 1980 Oct 1;204(1):10–17. doi: 10.1016/0003-9861(80)90002-8. [DOI] [PubMed] [Google Scholar]
- FORGET P., PICHINOTY F. INFLUENCE DE LA RESPIRATION ANA'EROBIE DU NITRATE ET DU FUMARATE SUR LE M'ETABOLISME FERMENTAIRE D'AEROBACTER AEROGENES. Biochim Biophys Acta. 1964 Feb 10;82:441–444. doi: 10.1016/0304-4165(64)90328-9. [DOI] [PubMed] [Google Scholar]
- HADJIPETROU L. P., STOUTHAMER A. H. ENERGY PRODUCTION DURING NITRATE RESPIRATION BY AEROBACTER AEROGENES. J Gen Microbiol. 1965 Jan;38:29–34. doi: 10.1099/00221287-38-1-29. [DOI] [PubMed] [Google Scholar]
- Ishimoto M., Shimokawa O. Reduction of trimethylamine N-oxide by Escherichia coli as anaerobic respiration. Z Allg Mikrobiol. 1978;18(3):173–181. doi: 10.1002/jobm.3630180304. [DOI] [PubMed] [Google Scholar]
- Kikuchi S., Ishimoto M. Nitrate respiration of Klebsiella pneumoniae on amino acids, especially on serine. Z Allg Mikrobiol. 1980;20(6):405–413. doi: 10.1002/jobm.3630200607. [DOI] [PubMed] [Google Scholar]
- Koike I., Hattori A. Energy yield of denitrification: an estimate from growth yield in continuous cultures of Pseudomonas denitrificans under nitrate-, nitrite- and oxide-limited conditions. J Gen Microbiol. 1975 May;88(1):11–19. doi: 10.1099/00221287-88-1-11. [DOI] [PubMed] [Google Scholar]
- Koike I., Hattori A. Growth yield of a denitrifying bacterium, Pseudomonas denitrificans, under aerobic and denitrifying conditions. J Gen Microbiol. 1975 May;88(1):1–10. doi: 10.1099/00221287-88-1-1. [DOI] [PubMed] [Google Scholar]
- Levin R. E. Correlation of DNA base composition and metabolism of Pseudomonas putrefaciens isolates from food, human clinical specimens, and other sources. Antonie Van Leeuwenhoek. 1972;38(2):121–127. doi: 10.1007/BF02328083. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Parker L. L., Levin R. E. Relative incidence of Alteromonas putrefaciens and Pseudomonas putrefaciens in ground beef. Appl Environ Microbiol. 1983 Mar;45(3):796–799. doi: 10.1128/aem.45.3.796-799.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. E., Weaver P. F. Fermentation and anaerobic respiration by Rhodospirillum rubrum and Rhodopseudomonas capsulata. J Bacteriol. 1982 Jan;149(1):181–190. doi: 10.1128/jb.149.1.181-190.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler W. J., Gilmour C. M. Biochemistry of nitrate respiration in Pseudomonas stutzeri. I. Aerobic and nitrate respiration routes of carbohydrate catabolism. J Bacteriol. 1966 Jan;91(1):245–250. doi: 10.1128/jb.91.1.245-250.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg E., Ringø E., Strøm A. R. Trimethylamine oxide respiration of Alteromonas putrefaciens NCMB 1735: Na+-stimulated anaerobic transport in cells and membrane vesicles. Appl Environ Microbiol. 1984 May;47(5):1090–1095. doi: 10.1128/aem.47.5.1090-1095.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg E., Styrvold O. B., Strøm A. R. Trimethylamine oxide respiration in Proteus sp. strain NTHC153: electron transfer-dependent phosphorylation and L-serine transport. J Bacteriol. 1982 Jan;149(1):22–28. doi: 10.1128/jb.149.1.22-28.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strøm A. R., Larsen H. Anaerobic fish spoilage by bacteria. I. Biochemical changes in herring extracts. J Appl Bacteriol. 1979 Jun;46(3):531–543. doi: 10.1111/j.1365-2672.1979.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Strøm A. R., Olafsen J. A., Larsen H. Trimethylamine oxide: a terminal electron acceptor in anaerobic respiration of bacteria. J Gen Microbiol. 1979 Jun;112(2):315–320. doi: 10.1099/00221287-112-2-315. [DOI] [PubMed] [Google Scholar]
- Takagi M., Tsuchiya T., Ishimoto M. Proton translocation coupled to trimethylamine N-oxide reduction in anaerobically grown Escherichia coli. J Bacteriol. 1981 Dec;148(3):762–768. doi: 10.1128/jb.148.3.762-768.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


