Abstract
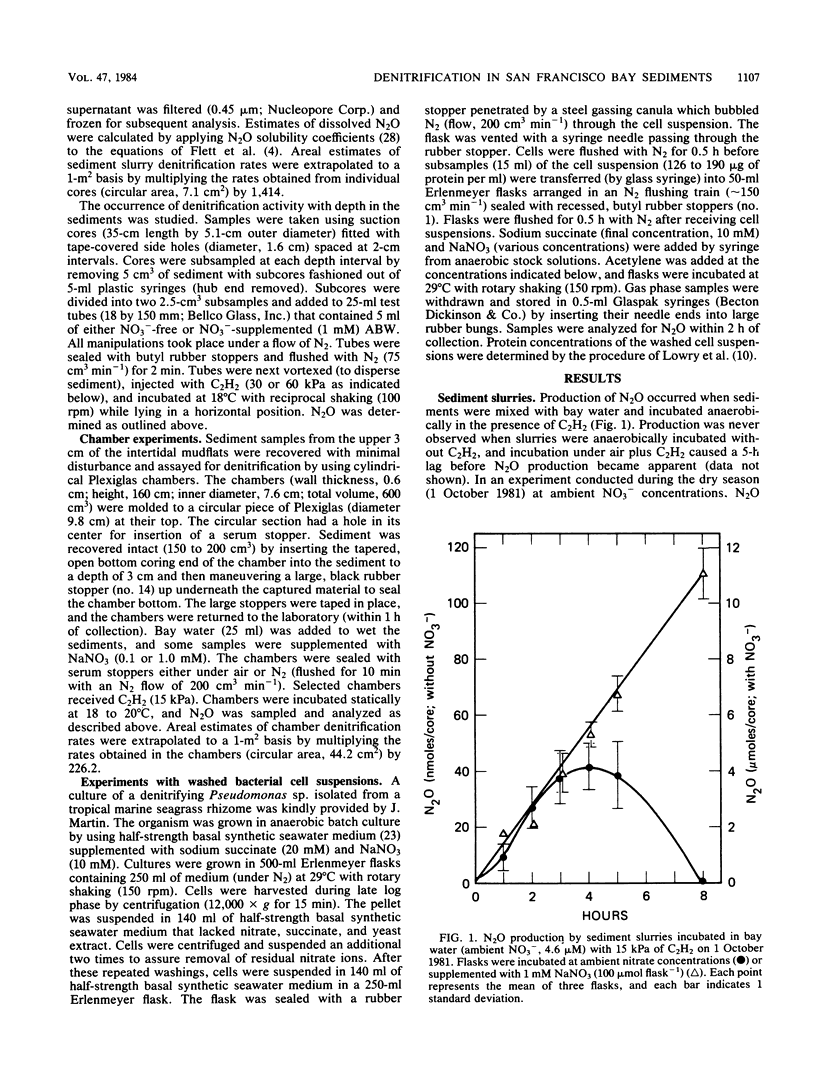
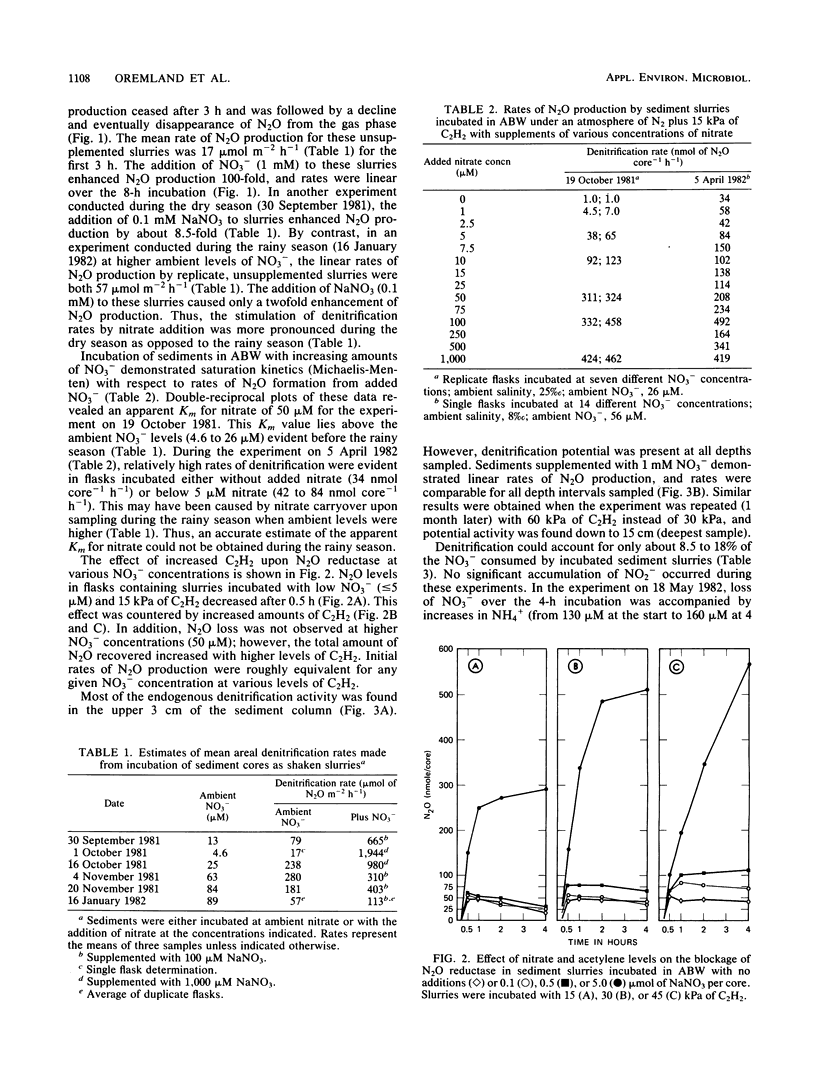
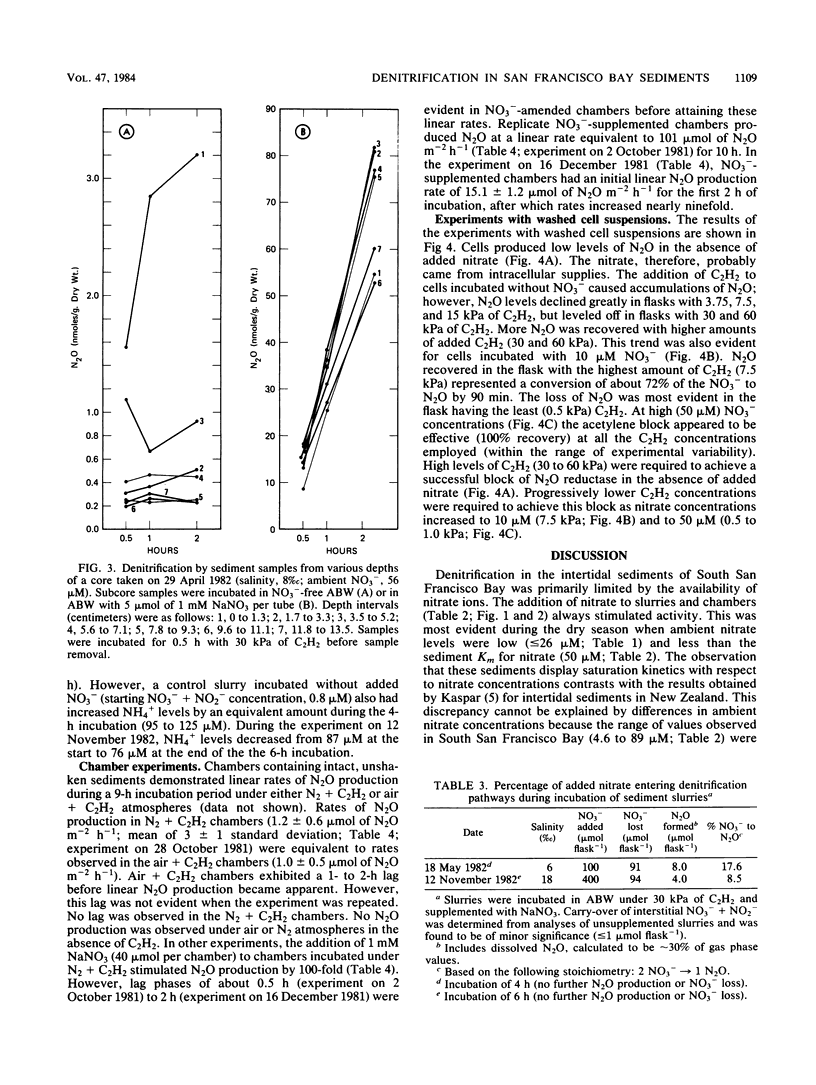
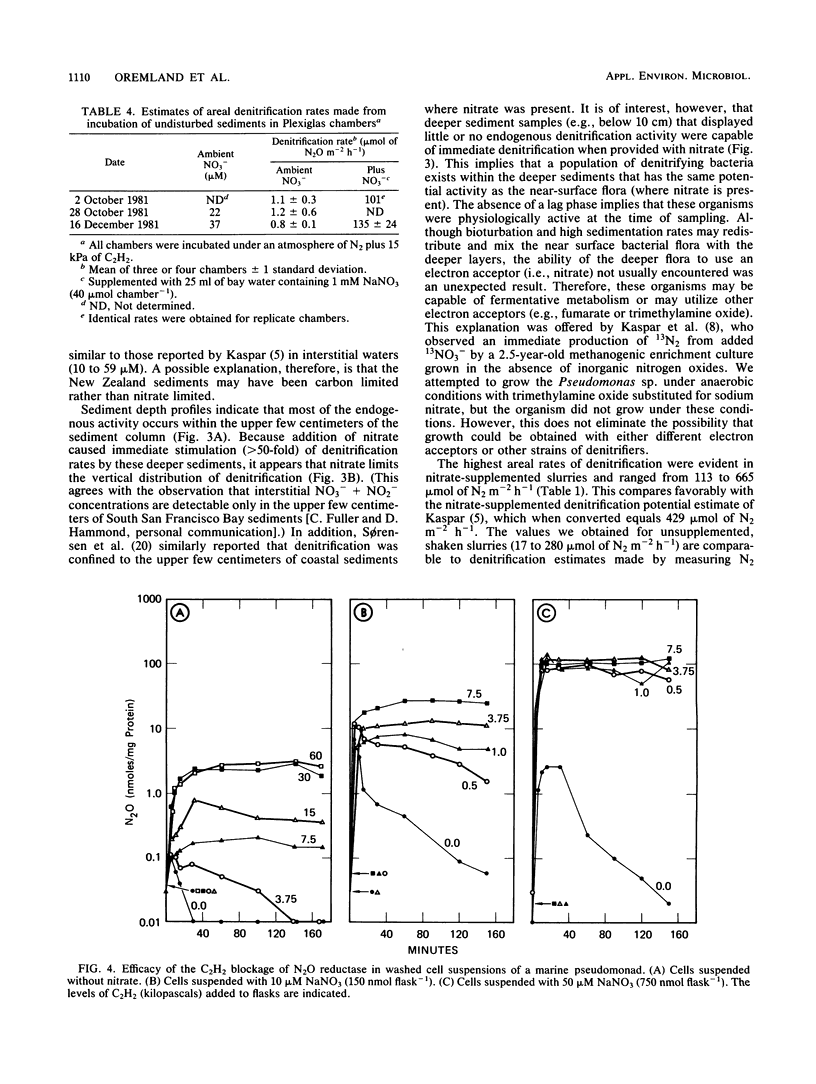
The acetylene block technique was employed to study denitrification in intertidal estuarine sediments. Addition of nitrate to sediment slurries stimulated denitrification. During the dry season, sediment-slurry denitrification rates displayed Michaelis-Menten kinetics, and ambient NO3− + NO2− concentrations (≤26 μM) were below the apparent Km (50 μM) for nitrate. During the rainy season, when ambient NO3− + NO2− concentrations were higher (37 to 89 μM), an accurate estimate of the Km could not be obtained. Endogenous denitrification activity was confined to the upper 3 cm of the sediment column. However, the addition of nitrate to deeper sediments demonstrated immediate N2O production, and potential activity existed at all depths sampled (the deepest was 15 cm). Loss of N2O in the presence of C2H2 was sometimes observed during these short-term sediment incubations. Experiments with sediment slurries and washed cell suspensions of a marine pseudomonad confirmed that this N2O loss was caused by incomplete blockage of N2O reductase by C2H2 at low nitrate concentrations. Areal estimates of denitrification (in the absence of added nitrate) ranged from 0.8 to 1.2 μmol of N2 m−2 h−1 (for undisturbed sediments) to 17 to 280 μmol of N2 m−2 h−1 (for shaken sediment slurries).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. K., Knowles R. Measurement of denitrification in two freshwater sediments by an in situ acetylene inhibition method. Appl Environ Microbiol. 1979 Jun;37(6):1067–1072. doi: 10.1128/aem.37.6.1067-1072.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson C. W., Zehnder A. J., Oremland R. S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981 Feb;41(2):396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flett R. J., Hamilton R. D., Campbell N. E. Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol. 1976 Jan;22(1):43–51. doi: 10.1139/m76-006. [DOI] [PubMed] [Google Scholar]
- Kaspar H. F. Denitrification in marine sediment: measurement of capacity and estimate of in situ rate. Appl Environ Microbiol. 1982 Mar;43(3):522–527. doi: 10.1128/aem.43.3.522-527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Tiedje J. M. Dissimilatory reduction of nitrate and nitrite in the bovine rumen: nitrous oxide production and effect of acetylene. Appl Environ Microbiol. 1981 Mar;41(3):705–709. doi: 10.1128/aem.41.3.705-709.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Tiedje J. M., Firestone R. B. Denitrification and dissimilatory nitrate reduction to ammonium in digested sludge. Can J Microbiol. 1981 Sep;27(9):878–885. doi: 10.1139/m81-139. [DOI] [PubMed] [Google Scholar]
- Knowles R. Denitrification, acetylene reduction, and methane metabolism in lake sediment exposed to acetylene. Appl Environ Microbiol. 1979 Sep;38(3):486–493. doi: 10.1128/aem.38.3.486-493.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nishio T., Koike I., Hattori A. Estimates of denitrification and nitrification in coastal and estuarine sediments. Appl Environ Microbiol. 1983 Feb;45(2):444–450. doi: 10.1128/aem.45.2.444-450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Capacity for denitrification and reduction of nitrate to ammonia in a coastal marine sediment. Appl Environ Microbiol. 1978 Feb;35(2):301–305. doi: 10.1128/aem.35.2.301-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Denitrification rates in a marine sediment as measured by the acetylene inhibition technique. Appl Environ Microbiol. 1978 Jul;36(1):139–143. doi: 10.1128/aem.36.1.139-143.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J., Tiedje J. M., Firestone R. B. Inhibition by sulfide of nitric and nitrous oxide reduction by denitrifying Pseudomonas fluorescens. Appl Environ Microbiol. 1980 Jan;39(1):105–108. doi: 10.1128/aem.39.1.105-108.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam T. Y., Knowles R. Effects of sulfide and acetylene on nitrous oxide reduction by soil and by Pseudomonas aeruginosa. Can J Microbiol. 1979 Oct;25(10):1133–1138. doi: 10.1139/m79-176. [DOI] [PubMed] [Google Scholar]
- Taylor B. F., Curry R. W., Corcoran E. F. Potential for biodegradation of phthalic Acid esters in marine regions. Appl Environ Microbiol. 1981 Oct;42(4):590–595. doi: 10.1128/aem.42.4.590-595.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triska F. J., Oremland R. S. Denitrification associated with periphyton communities. Appl Environ Microbiol. 1981 Oct;42(4):745–748. doi: 10.1128/aem.42.4.745-748.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]


