Abstract
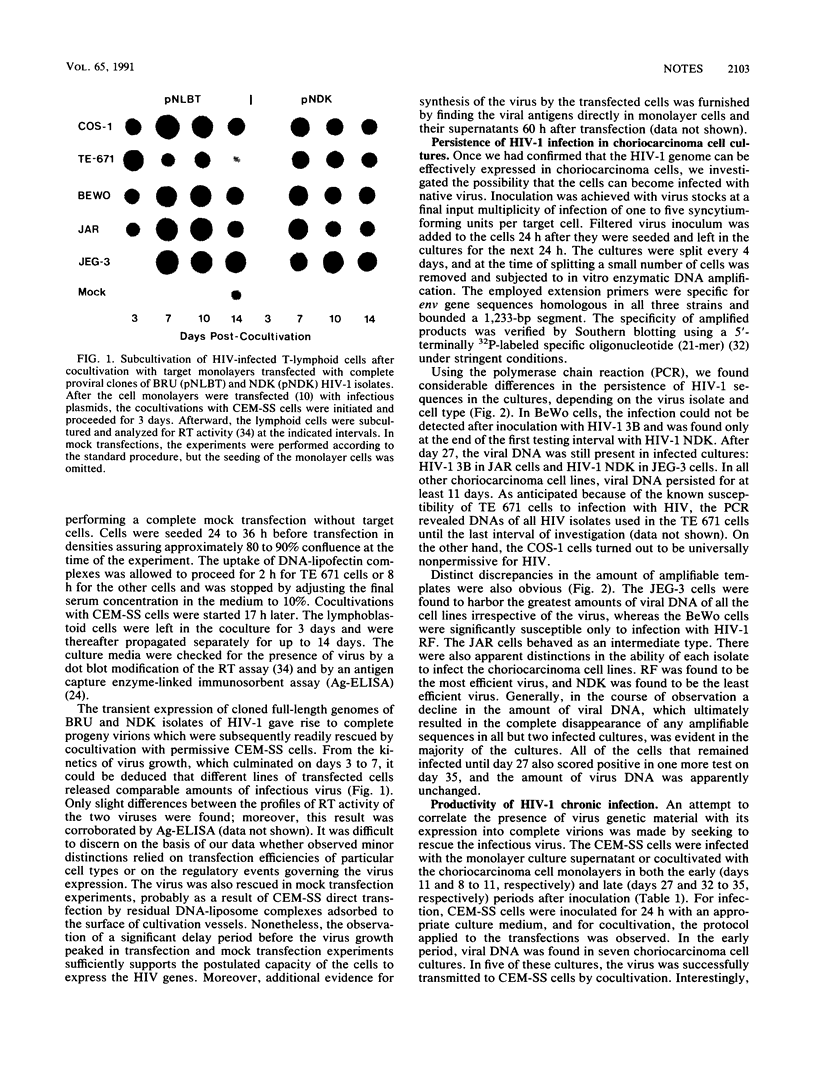
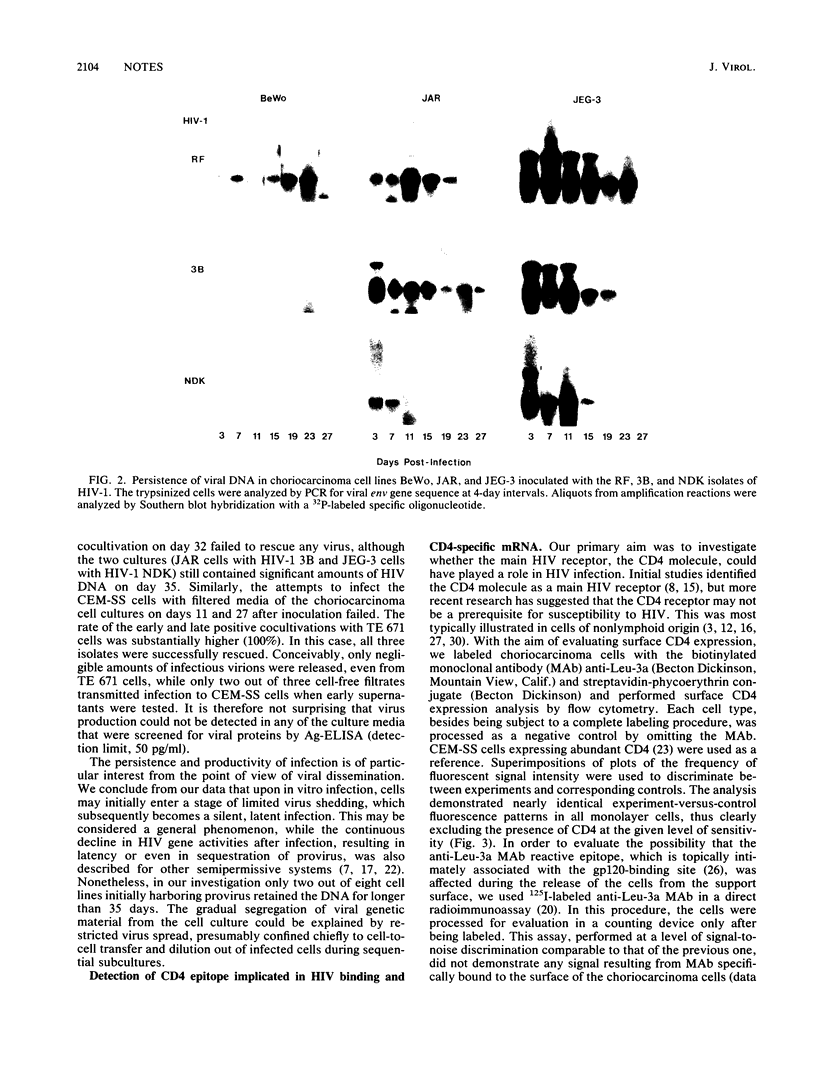
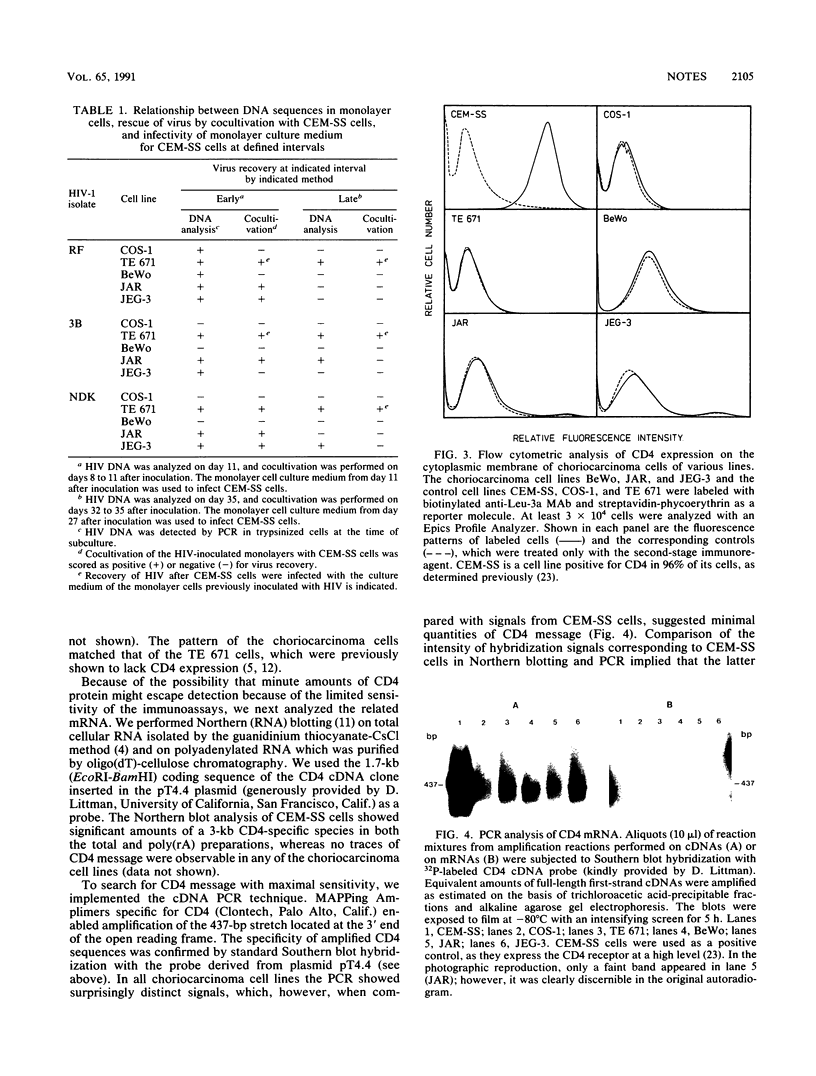
We investigated the nature of interaction of the malignantly transformed cell lines of trophoblast origin BeWo, JAR, and JEG-3 with three different human immunodeficiency virus type 1 (HIV-1) isolates (RF, 3B, and NDK). After inoculation with cell-free virus, the persistence of infection was determined for 1 month by monitoring the presence of viral DNA in the cells by the polymerase chain reaction (PCR). Furthermore, the infectious virus in the culture supernatant was assayed with CEM-SS cells, and attempts to rescue the virus by cocultivation with CEM-SS cells were made. Appraised on the basis of the relative amount of viral DNA and the frequency of positive cocultivation. JEG-3 was the most permissive and BeWo was the least permissive cell line. However, when the cells were transfected with two biologically active molecular clones of HIV-1, the BRU and NDK isolates, all three cell lines turned out to support the production of mature virus progeny to the same extent. The abundance of viral DNA sequences in the infected cells varied with the isolate, showing an overall decline from RF to NDK. The amount of viral DNA in the cells and its expression decreased during the period of observation; this decrease was mirrored in an erosion of the virus recovery rate at cocultivation from 71% recovery on day 8 to failure of isolation on day 32. None of the cell lines expressed detectable amounts of cell surface CD4 molecules when assayed by flow microfluorometry and direct radioimmunoassay. Northern (RNA) blot hybridization analysis of both the total RNA and the mRNA did not reveal any CD4-specific message: nonetheless, by using the PCR, sequences specifically related to the CD4 gene were uncovered. The data demonstrate that the trophoblast-derived cell lines are susceptible to infection with HIV and that they support transient viral replication in the initial phases of infection. However, the latent form of infection may persist over a period of several weeks.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Gendelman H. E., Koenig S., Folks T., Willey R., Rabson A., Martin M. A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986 Aug;59(2):284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizon M., Sonigo P., Barré-Sinoussi F., Chermann J. C., Tiollais P., Montagnier L., Wain-Hobson S. Molecular cloning of lymphadenopathy-associated virus. Nature. 1984 Dec 20;312(5996):757–760. doi: 10.1038/312757a0. [DOI] [PubMed] [Google Scholar]
- Cao Y. Z., Friedman-Kien A. E., Huang Y. X., Li X. L., Mirabile M., Moudgil T., Zucker-Franklin D., Ho D. D. CD4-independent, productive human immunodeficiency virus type 1 infection of hepatoma cell lines in vitro. J Virol. 1990 Jun;64(6):2553–2559. doi: 10.1128/jvi.64.6.2553-2559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clapham P. R., Weber J. N., Whitby D., McIntosh K., Dalgleish A. G., Maddon P. J., Deen K. C., Sweet R. W., Weiss R. A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- Dahl K. E., Burrage T., Jones F., Miller G. Persistent nonproductive infection of Epstein-Barr virus-transformed human B lymphocytes by human immunodeficiency virus type 1. J Virol. 1990 Apr;64(4):1771–1783. doi: 10.1128/jvi.64.4.1771-1783.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl K., Martin K., Miller G. Differences among human immunodeficiency virus strains in their capacities to induce cytolysis or persistent infection of a lymphoblastoid cell line immortalized by Epstein-Barr virus. J Virol. 1987 May;61(5):1602–1608. doi: 10.1128/jvi.61.5.1602-1608.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Ellrodt A., Barre-Sinoussi F., Le Bras P., Nugeyre M. T., Palazzo L., Rey F., Brun-Vezinet F., Rouzioux C., Segond P., Caquet R. Isolation of human T-lymphotropic retrovirus (LAV) from Zairian married couple, one with AIDS, one with prodromes. Lancet. 1984 Jun 23;1(8391):1383–1385. doi: 10.1016/s0140-6736(84)91877-4. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harouse J. M., Kunsch C., Hartle H. T., Laughlin M. A., Hoxie J. A., Wigdahl B., Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A. D., Banapour B., Levy J. A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985 Dec;147(2):326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- Jovaisas E., Koch M. A., Schäfer A., Stauber M., Löwenthal D. LAV/HTLV-III in 20-week fetus. Lancet. 1985 Nov 16;2(8464):1129–1129. doi: 10.1016/s0140-6736(85)90720-2. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kunsch C., Hartle H. T., Wigdahl B. Infection of human fetal dorsal root ganglion glial cells with human immunodeficiency virus type 1 involves an entry mechanism independent of the CD4 T4A epitope. J Virol. 1989 Dec;63(12):5054–5061. doi: 10.1128/jvi.63.12.5054-5061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunsch C., Wigdahl B. Transient expression of human immunodeficiency virus type 1 genome results in a nonproductive infection in human fetal dorsal root ganglia glial cells. Virology. 1989 Dec;173(2):715–722. doi: 10.1016/0042-6822(89)90585-0. [DOI] [PubMed] [Google Scholar]
- Lewis S. H., Reynolds-Kohler C., Fox H. E., Nelson J. A. HIV-1 in trophoblastic and villous Hofbauer cells, and haematological precursors in eight-week fetuses. Lancet. 1990 Mar 10;335(8689):565–568. doi: 10.1016/0140-6736(90)90349-a. [DOI] [PubMed] [Google Scholar]
- Maury W., Potts B. J., Rabson A. B. HIV-1 infection of first-trimester and term human placental tissue: a possible mode of maternal-fetal transmission. J Infect Dis. 1989 Oct;160(4):583–588. doi: 10.1093/infdis/160.4.583. [DOI] [PubMed] [Google Scholar]
- McKeating J. A., Griffiths P. D., Weiss R. A. HIV susceptibility conferred to human fibroblasts by cytomegalovirus-induced Fc receptor. Nature. 1990 Feb 15;343(6259):659–661. doi: 10.1038/343659a0. [DOI] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Monroe J. E., Calender A., Mulder C. Epstein-Barr virus-positive and -negative B-cell lines can be infected with human immunodeficiency virus types 1 and 2. J Virol. 1988 Sep;62(9):3497–3500. doi: 10.1128/jvi.62.9.3497-3500.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara P. L., Hatch W. C., Dunlop N. M., Robey W. G., Arthur L. O., Gonda M. A., Fischinger P. J. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retroviruses. 1987 Fall;3(3):283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- Nielsen C. M., Bygbjerg I. C., Vestergaard B. F. Detection of HIV antigens in eluates from whole blood collected on filterpaper. Lancet. 1987 Mar 7;1(8532):566–567. doi: 10.1016/s0140-6736(87)90213-3. [DOI] [PubMed] [Google Scholar]
- Rey F., Barré-Sinoussi F., Schmidtmayerova H., Chermann J. C. Detection and titration of neutralizing antibodies to HIV using an inhibition of the cytopathic effect of the virus on MT4 cells. J Virol Methods. 1987 Jun;16(3):239–249. doi: 10.1016/0166-0934(87)90008-5. [DOI] [PubMed] [Google Scholar]
- Sattentau Q. J., Arthos J., Deen K., Hanna N., Healey D., Beverley P. C., Sweet R., Truneh A. Structural analysis of the human immunodeficiency virus-binding domain of CD4. Epitope mapping with site-directed mutants and anti-idiotypes. J Exp Med. 1989 Oct 1;170(4):1319–1334. doi: 10.1084/jem.170.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spire B., Sire J., Zachar V., Rey F., Barré-Sinoussi F., Galibert F., Hampe A., Chermann J. C. Nucleotide sequence of HIV1-NDK: a highly cytopathic strain of the human immunodeficiency virus. Gene. 1989 Sep 30;81(2):275–284. doi: 10.1016/0378-1119(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Sprecher S., Soumenkoff G., Puissant F., Degueldre M. Vertical transmission of HIV in 15-week fetus. Lancet. 1986 Aug 2;2(8501):288–289. doi: 10.1016/s0140-6736(86)92110-0. [DOI] [PubMed] [Google Scholar]
- Tateno M., Gonzalez-Scarano F., Levy J. A. Human immunodeficiency virus can infect CD4-negative human fibroblastoid cells. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4287–4290. doi: 10.1073/pnas.86.11.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth F. D., Juhl C., Nørskov-Lauritsen N., Mosborg Petersen P., Ebbesen P. Interferon production by cultured human trophoblast induced with double stranded polyribonucleotide. J Reprod Immunol. 1990 Jun;17(3):217–227. doi: 10.1016/0165-0378(90)90004-p. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Miyada C. G. Oligonucleotide probes for the screening of recombinant DNA libraries. Methods Enzymol. 1987;152:432–442. doi: 10.1016/0076-6879(87)52050-x. [DOI] [PubMed] [Google Scholar]
- White T. E., Saltzman R. A., Di Sant'Agnese P. A., Keng P. C., Sutherland R. M., Miller R. K. Human choriocarcinoma (JAr) cells grown as multicellular spheroids. Placenta. 1988 Nov-Dec;9(6):583–598. doi: 10.1016/0143-4004(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]