Abstract
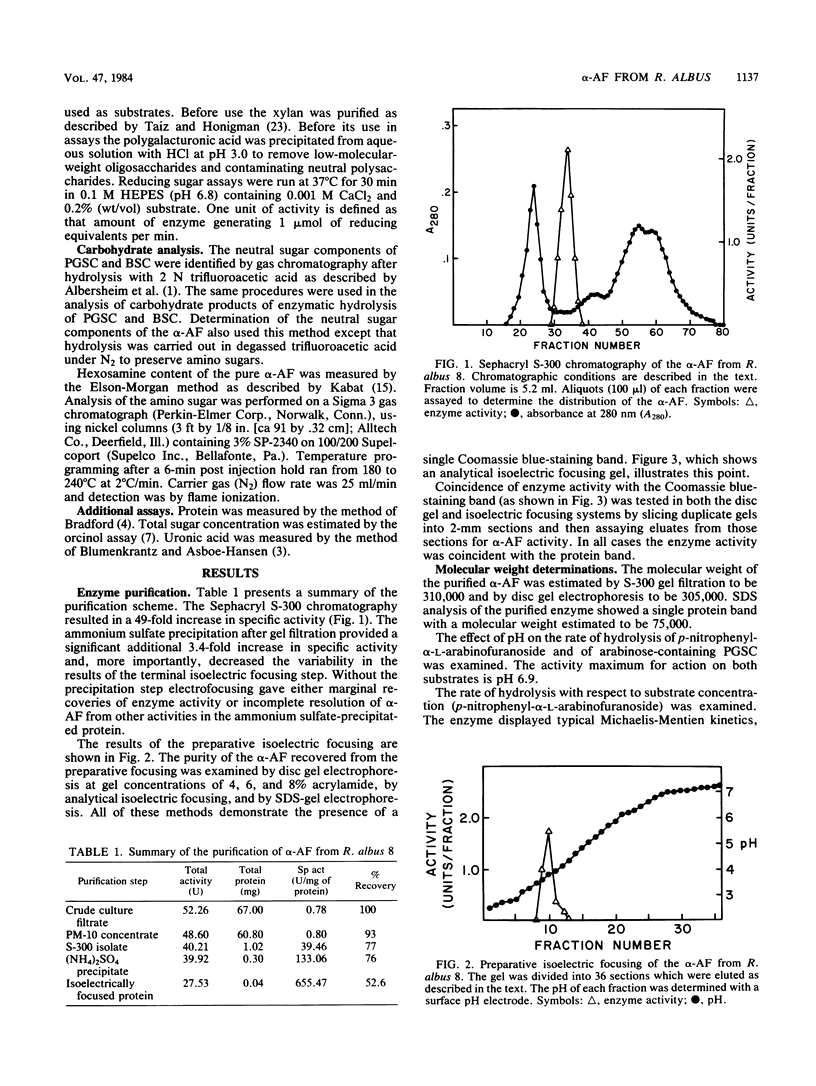
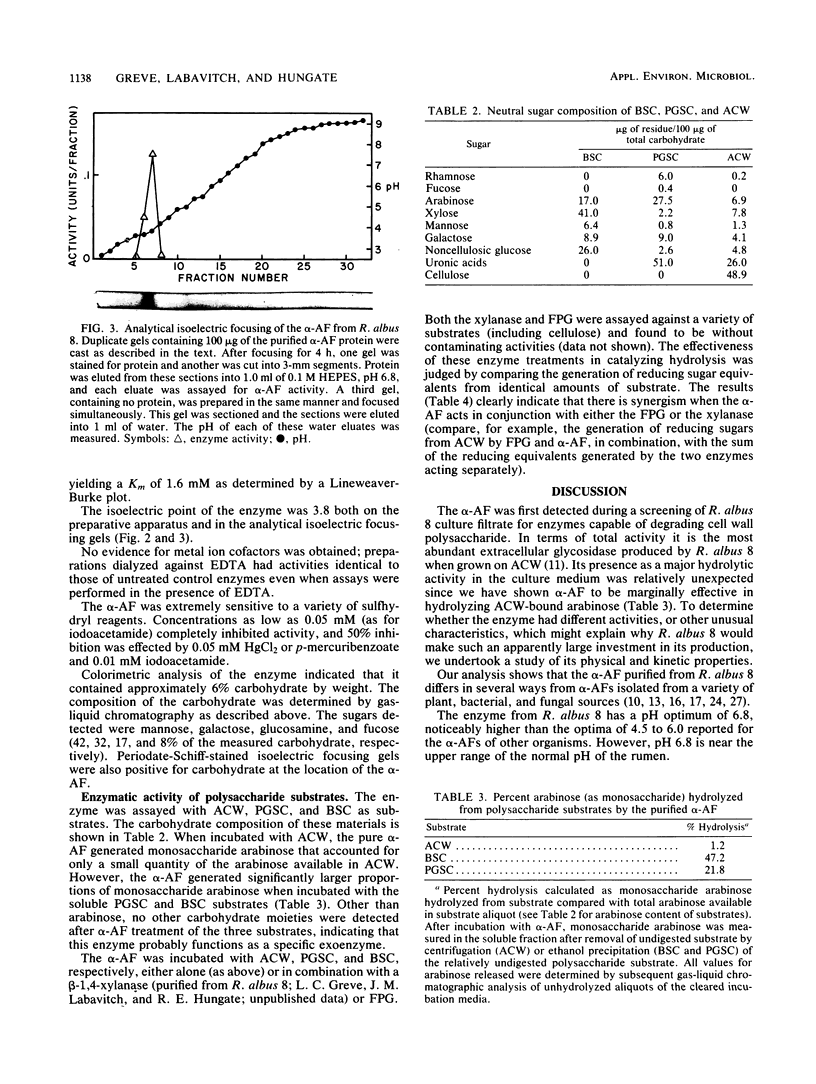
An alpha-L- arabinofuranosidase has been purified from the extracellular broth of cultures of Ruminococcus albus 8. The purification procedure utilized gel filtration, (NH4)2SO4 precipitation, and isoelectric focusing. The purified enzyme appeared to be homogeneous when chromatographed on disc and analytical isoelectric focusing gels. The estimated molecular weight of the native protein was 305,000 to 310,000. Sodium dodecyl sulfate-gel electrophoresis analysis suggested that the native protein is a tetramer composed of 75,000-molecular-weight subunits. The enzyme appeared to have no metal cofactor requirement but was sensitive to several sulfhydryl reagents. The pH optimum with p-nitrophenyl-alpha-L-arabinofuranoside as the substrate was 6.9 and the Km was 1.3 mM. Several lines of evidence indicated that the enzyme is a glycoprotein. When assayed against alfalfa cell wall material, the enzyme hydrolyzed only small amounts of arabinose from the substrate. When assayed together with hemicellulolytic or pectinolytic enzymes against the same substrate, the arabinosidase significantly enhanced the hydrolytic action of the glycanases .
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Dehority B. A. Hemicellulose degradation by rumen bacteria. Fed Proc. 1973 Jul;32(7):1819–1825. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fielding A. H., Byrde R. J. The partial purification and properties of endopolygalacturonase and alpha-L-arabino-furanosidase secreted by Sclerontinia fructigena. J Gen Microbiol. 1969 Sep;58(1):73–84. doi: 10.1099/00221287-58-1-73. [DOI] [PubMed] [Google Scholar]
- Greve L. C., Labavitch J. M., Stack R. J., Hungate R. E. Muralytic Activities of Ruminococcus albus 8. Appl Environ Microbiol. 1984 May;47(5):1141–1145. doi: 10.1128/aem.47.5.1141-1145.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hungate R. E., Stack R. J. Phenylpropanoic Acid: Growth Factor for Ruminococcus albus. Appl Environ Microbiol. 1982 Jul;44(1):79–83. doi: 10.1128/aem.44.1.79-83.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. II. Preparative isoelectric focusing. Biochim Biophys Acta. 1975 Mar 28;386(1):181–195. doi: 10.1016/0005-2795(75)90258-5. [DOI] [PubMed] [Google Scholar]
- Righetti P. G., Drysdale J. W. Isoelectric focusing in gels. J Chromatogr. 1974 Sep 25;98(2):271–321. doi: 10.1016/s0021-9673(00)92076-4. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Taiz L., Honigman W. A. Production of cell wall hydrolyzing enzymes by barley aleurone layers in response to gibberellic Acid. Plant Physiol. 1976 Sep;58(3):380–386. doi: 10.1104/pp.58.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Uchida T. Purification and properties of alpha-l-arabinofuranosidase from plant Scopolia japonica calluses. Biochim Biophys Acta. 1978 Feb 10;522(2):531–540. doi: 10.1016/0005-2744(78)90085-2. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weinstein L., Albersheim P. Structure of Plant Cell Walls: IX. Purification and Partial Characterization of a Wall-degrading Endo-Arabanase and an Arabinosidase from Bacillus subtilis. Plant Physiol. 1979 Mar;63(3):425–432. doi: 10.1104/pp.63.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley C. W. Analytical fractionation of plant and animal proteins by gel electrofocusing. J Chromatogr. 1968 Aug 27;36(3):362–365. doi: 10.1016/s0021-9673(01)92959-0. [DOI] [PubMed] [Google Scholar]