Abstract
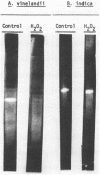
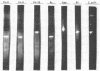
Quantitative N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) oxidase and superoxide dismutase (SOD) analyses were performed on representative organisms of the family Azotobacteraceae. Azotobacter vinelandii, Azotobacter chroococcum, Azotobacter paspali, and Derxia gummosa exhibited high quantitative TMPD oxidase activities, and their extracts possessed very active and electrophoretically homogeneous (single gel band) Fe-type SODs. Azomonas macrocytogenes extracts had similar single Fe-type SODs, and their cells exhibited no TMPD-dependent cytochrome oxidase activity. Nitrogen-fixing cells of Beijerinckia indica, Beijerinckia derxii, and Beijerinckia mobilis exhibited minimal TMPD oxidation capabilities (rates equivalent to the TMPD autooxidation reaction), and these extracts also possessed very active SODs but only of the Mn metallotype.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada K., Yoshikawa K., Takahashi M., Maeda Y., Enmanji K. Superoxide dismutases from a blue-green alga, Plectonema boryanum. J Biol Chem. 1975 Apr 25;250(8):2801–2807. [PubMed] [Google Scholar]
- Barrette W. C., Jr, Sawyer D. T., Fee J. A., Asada K. Potentiometric titrations and oxidation--reduction potentials of several iron superoxide dismutases. Biochemistry. 1983 Feb 1;22(3):624–627. doi: 10.1021/bi00272a015. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Britton L., Malinowski D. P., Fridovich I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J Bacteriol. 1978 Apr;134(1):229–236. doi: 10.1128/jb.134.1.229-236.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan A. G., Lees H. Superoxide dismutase from nitrogen-fixing Azotobacter chroococcum: purification, characterization, and intracellular location. Can J Microbiol. 1980 Apr;26(4):441–447. doi: 10.1139/m80-073. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Biochemical mechanisms accounting for the toxic action of oxygen on living organisms: the key role of superoxide dismutase. Cell Biol Int Rep. 1978 Mar;2(2):113–128. doi: 10.1016/0309-1651(78)90032-2. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Aston P. R., Old L. Enzymatic oxidation of tetramethyl-p-phenylenediamine and p-phenylenediamine by the electron transport particulate fraction of Azotobacter vinelandii. J Bacteriol. 1967 Mar;93(3):1069–1078. doi: 10.1128/jb.93.3.1069-1078.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Jr, Marcucci O. M., McQuitty D. N. Tetramethyl-p-phenylenediamine oxidase reaction in Azotobacter vinelandii. Appl Microbiol. 1975 Dec;30(6):951–958. doi: 10.1128/am.30.6.951-958.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Manning S., Barrera C. R. Isolation and purification of the D(-)beta-hydroxybutyric dehydrogenase of Azotobacter vinelandii. Can J Microbiol. 1968 Jul;14(7):775–783. doi: 10.1139/m68-129. [DOI] [PubMed] [Google Scholar]
- Lieberman M. M., Lanyi J. K. Studies of the electron transport chain of extremely halophilic bacteria. V. Mode of action of salts on cytochrome oxidase. Biochim Biophys Acta. 1971 Aug 6;245(1):21–33. doi: 10.1016/0005-2728(71)90004-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Postgate J. R. Biological nitrogen fixation. Nature. 1970 Apr 4;226(5240):25–27. doi: 10.1038/226025a0. [DOI] [PubMed] [Google Scholar]