Abstract
The fusion glycoprotein precursor of Newcastle disease virus is ubiquitously cleaved in the constitutive secretory pathway if it possesses an oligobasic cleavage motif (RRQR/KR), whereas the precursor is refractory to cleavage if the motif is monobasic (GR/KQGR). We examined the cleavage activity of the mammalian subtilisin-related proteinases furin/PACE, PC2, and PC1/PC3, which are thought to be responsible for proprotein processing in either the constitutive (furin/PACE) or the regulated (PC2 and PC1/PC3) secretory pathway, for the viral precursors with different cleavage motifs. Only furin/PACE was fully capable of cleaving the precursors with the oligobasic motif. PC2 and PC1/PC3 were incapable or only partially capable of cleaving at this motif. None of the proteinases cleaved the monobasic motif. These results suggest involvement of furin/PACE in viral protein processing in the constitutive secretory pathway.
Full text
PDF

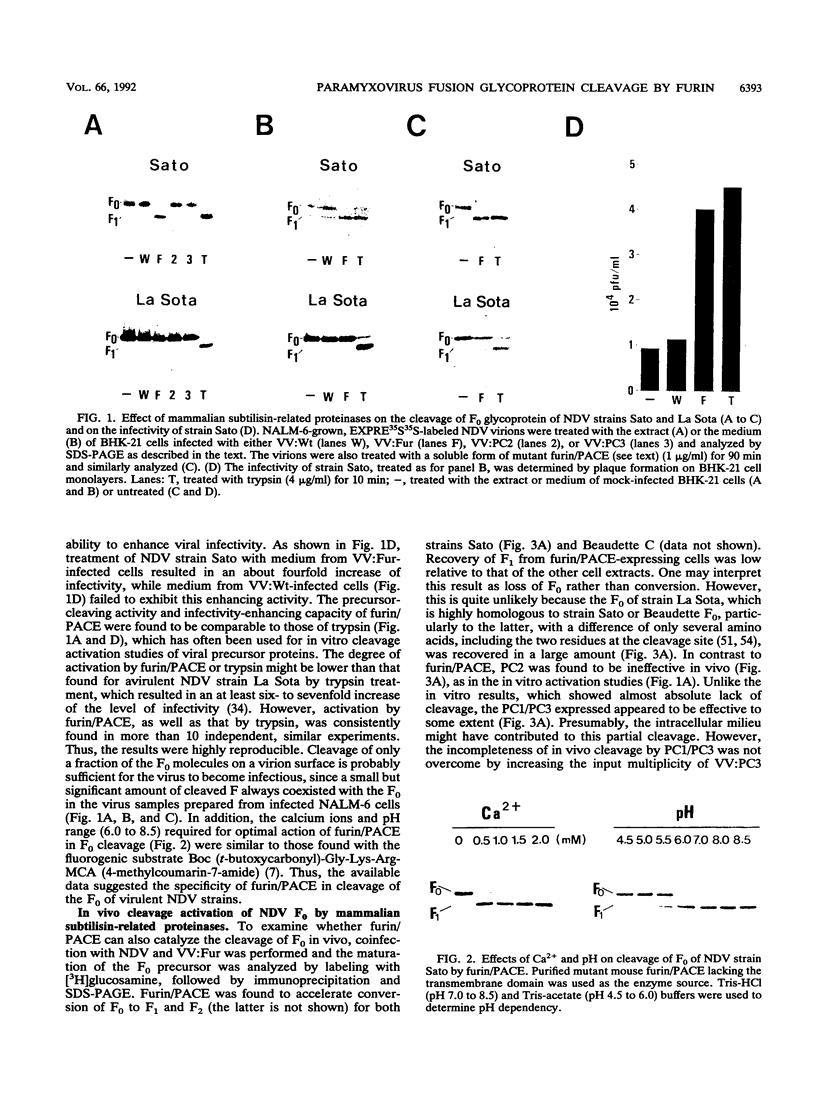



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abenes G., Kida H., Yanagawa R. Antigenic mapping and functional analysis of the F protein of Newcastle disease virus using monoclonal antibodies. Arch Virol. 1986;90(1-2):97–110. doi: 10.1007/BF01314148. [DOI] [PubMed] [Google Scholar]
- Barr P. J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991 Jul 12;66(1):1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- Bentley A. K., Rees D. J., Rizza C., Brownlee G. G. Defective propeptide processing of blood clotting factor IX caused by mutation of arginine to glutamine at position -4. Cell. 1986 May 9;45(3):343–348. doi: 10.1016/0092-8674(86)90319-3. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Garten W., Klenk H. D., Rott R. Proteolytic cleavage of influenza virus hemagglutinins: primary structure of the connecting peptide between HA1 and HA2 determines proteolytic cleavability and pathogenicity of Avian influenza viruses. Virology. 1981 Sep;113(2):725–735. doi: 10.1016/0042-6822(81)90201-4. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Orlich M., Klenk H. D., Rott R. The structure of the hemagglutinin, a determinant for the pathogenicity of influenza viruses. Virology. 1979 May;95(1):197–207. doi: 10.1016/0042-6822(79)90414-8. [DOI] [PubMed] [Google Scholar]
- Brenner C., Fuller R. S. Structural and enzymatic characterization of a purified prohormone-processing enzyme: secreted, soluble Kex2 protease. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):922–926. doi: 10.1073/pnas.89.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan P. A., Leduc R., Thomas L., Thorner J., Gibson H. L., Brake A. J., Barr P. J., Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990 Dec;111(6 Pt 2):2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Brake A. J., Thorner J. Intracellular targeting and structural conservation of a prohormone-processing endoprotease. Science. 1989 Oct 27;246(4929):482–486. doi: 10.1126/science.2683070. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Brake A., Thorner J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Sterne R. E., Thorner J. Enzymes required for yeast prohormone processing. Annu Rev Physiol. 1988;50:345–362. doi: 10.1146/annurev.ph.50.030188.002021. [DOI] [PubMed] [Google Scholar]
- Gething M. J., White J. M., Waterfield M. D. Purification of the fusion protein of Sendai virus: analysis of the NH2-terminal sequence generated during precursor activation. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2737–2740. doi: 10.1073/pnas.75.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman R. L., Syddall R. J., Iorio R. M., Sheehan J. P., Bratt M. A. Quantitative basic residue requirements in the cleavage-activation site of the fusion glycoprotein as a determinant of virulence for Newcastle disease virus. J Virol. 1988 Jan;62(1):354–356. doi: 10.1128/jvi.62.1.354-356.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh B., Ogasawara T., Toyoda T., Inocencio N. M., Hamaguchi M., Nagai Y. An endoprotease homologous to the blood clotting factor X as a determinant of viral tropism in chick embryo. EMBO J. 1990 Dec;9(12):4189–4195. doi: 10.1002/j.1460-2075.1990.tb07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh B., Yamauchi F., Ogasawara T., Nagai Y. Isolation of factor Xa from chick embryo as the amniotic endoprotease responsible for paramyxovirus activation. FEBS Lett. 1992 Jan 27;296(3):274–278. doi: 10.1016/0014-5793(92)80303-x. [DOI] [PubMed] [Google Scholar]
- Hakes D. J., Birch N. P., Mezey A., Dixon J. E. Isolation of two complementary deoxyribonucleic acid clones from a rat insulinoma cell line based on similarities to Kex2 and furin sequences and the specific localization of each transcript to endocrine and neuroendocrine tissues in rats. Endocrinology. 1991 Dec;129(6):3053–3063. doi: 10.1210/endo-129-6-3053. [DOI] [PubMed] [Google Scholar]
- Hatsuzawa K., Hosaka M., Nakagawa T., Nagase M., Shoda A., Murakami K., Nakayama K. Structure and expression of mouse furin, a yeast Kex2-related protease. Lack of processing of coexpressed prorenin in GH4C1 cells. J Biol Chem. 1990 Dec 25;265(36):22075–22078. [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M., Nagahama M., Kim W. S., Watanabe T., Hatsuzawa K., Ikemizu J., Murakami K., Nakayama K. Arg-X-Lys/Arg-Arg motif as a signal for precursor cleavage catalyzed by furin within the constitutive secretory pathway. J Biol Chem. 1991 Jul 5;266(19):12127–12130. [PubMed] [Google Scholar]
- Julius D., Brake A., Blair L., Kunisawa R., Thorner J. Isolation of the putative structural gene for the lysine-arginine-cleaving endopeptidase required for processing of yeast prepro-alpha-factor. Cell. 1984 Jul;37(3):1075–1089. doi: 10.1016/0092-8674(84)90442-2. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R. The molecular biology of influenza virus pathogenicity. Adv Virus Res. 1988;34:247–281. doi: 10.1016/S0065-3527(08)60520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J., Chun J., Harter D., Axel R. Isolation and functional expression of a mammalian prohormone processing enzyme, murine prohormone convertase 1. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6834–6838. doi: 10.1073/pnas.88.15.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Oda K., Fujiwara T., Takami N., Tashiro K., Ikehara Y. Functional expression of furin demonstrating its intracellular localization and endoprotease activity for processing of proalbumin and complement pro-C3. J Biol Chem. 1991 Sep 5;266(25):16954–16959. [PubMed] [Google Scholar]
- Misumi Y., Sohda M., Ikehara Y. Sequence of the cDNA encoding rat furin, a possible propeptide-processing endoprotease. Nucleic Acids Res. 1990 Nov 25;18(22):6719–6719. doi: 10.1093/nar/18.22.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Nakamura T., Ohshima T., Tanaka S., Matsuo H. Characterization of KEX2-encoded endopeptidase from yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1989 Feb 28;159(1):305–311. doi: 10.1016/0006-291x(89)92438-8. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Nakamura T., Ohshima T., Tanaka S., Matsuo H. Yeast KEX2 genes encodes an endopeptidase homologous to subtilisin-like serine proteases. Biochem Biophys Res Commun. 1988 Oct 14;156(1):246–254. doi: 10.1016/s0006-291x(88)80832-5. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Nakamura T., Takada K., Sakakibara S., Matsuo H. A membrane-bound, calcium-dependent protease in yeast alpha-cell cleaving on the carboxyl side of paired basic residues. Biochem Biophys Res Commun. 1987 Apr 29;144(2):807–814. doi: 10.1016/s0006-291x(87)80036-0. [DOI] [PubMed] [Google Scholar]
- Morrison T., Ward L. J., Semerjian A. Intracellular processing of the Newcastle disease virus fusion glycoprotein. J Virol. 1985 Mar;53(3):851–857. doi: 10.1128/jvi.53.3.851-857.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Hamaguchi M., Toyoda T. Molecular biology of Newcastle disease virus. Prog Vet Microbiol Immunol. 1989;5:16–64. [PubMed] [Google Scholar]
- Nagai Y., Inocencio N. M., Gotoh B. Paramyxovirus tropism dependent on host proteases activating the viral fusion glycoprotein. Behring Inst Mitt. 1991 Jul;(89):35–45. [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D. Activation of precursors to both glycoporteins of Newcastle disease virus by proteolytic cleavage. Virology. 1977 Mar;77(1):125–134. doi: 10.1016/0042-6822(77)90412-3. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Ogura H., Klenk H. Studies on the assembly of the envelope of Newcastle disease virus. Virology. 1976 Feb;69(2):523–538. doi: 10.1016/0042-6822(76)90482-7. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Shimokata K., Yoshida T., Hamaguchi M., Iinuma M., Maeno K., Matsumoto T., Klenk H. D., Rott R. The spread of a pathogenic and an apathogenic strain of Newcastle disease virus in the chick embryo as depending on the protease sensitivity of the virus glycoproteins. J Gen Virol. 1979 Nov;45(2):263–272. doi: 10.1099/0022-1317-45-2-263. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Hosaka M., Hatsuzawa K., Murakami K. Cloning and functional expression of a novel endoprotease involved in prohormone processing at dibasic sites. J Biochem. 1991 Jun;109(6):803–806. doi: 10.1093/oxfordjournals.jbchem.a123461. [DOI] [PubMed] [Google Scholar]
- Naruse H., Nagai Y., Yoshida T., Hamaguchi M., Matsumoto T., Isomura S., Suzuki S. The polypeptides of mumps virus and their synthesis in infected chick embryo cells. Virology. 1981 Jul 15;112(1):119–130. doi: 10.1016/0042-6822(81)90618-8. [DOI] [PubMed] [Google Scholar]
- Ogasawara T., Gotoh B., Suzuki H., Asaka J., Shimokata K., Rott R., Nagai Y. Expression of factor X and its significance for the determination of paramyxovirus tropism in the chick embryo. EMBO J. 1992 Feb;11(2):467–472. doi: 10.1002/j.1460-2075.1992.tb05076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroek A. J., Schalken J. A., Leunissen J. A., Onnekink C., Bloemers H. P., Van de Ven W. J. Evolutionary conserved close linkage of the c-fes/fps proto-oncogene and genetic sequences encoding a receptor-like protein. EMBO J. 1986 Sep;5(9):2197–2202. doi: 10.1002/j.1460-2075.1986.tb04484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T., Matsuda Y., Kiyokage R., Kawahara N., Kiyotani K., Katunuma N., Nagai Y., Yoshida T. Identification of endoprotease activity in the trans Golgi membranes of rat liver cells that specifically processes in vitro the fusion glycoprotein precursor of virulent Newcastle disease virus. Virology. 1991 Oct;184(2):504–512. doi: 10.1016/0042-6822(91)90420-g. [DOI] [PubMed] [Google Scholar]
- Schalken J. A., Roebroek A. J., Oomen P. P., Wagenaar S. S., Debruyne F. M., Bloemers H. P., Van de Ven W. J. fur gene expression as a discriminating marker for small cell and nonsmall cell lung carcinomas. J Clin Invest. 1987 Dec;80(6):1545–1549. doi: 10.1172/JCI113240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gaspar L., Mion P., Marcinkiewicz M., Mbikay M., Chrétien M. cDNA sequence of two distinct pituitary proteins homologous to Kex2 and furin gene products: tissue-specific mRNAs encoding candidates for pro-hormone processing proteinases. DNA Cell Biol. 1990 Jul-Aug;9(6):415–424. doi: 10.1089/dna.1990.9.415. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Marcinkiewicz M., Benjannet S., Gaspar L., Beaubien G., Mattei M. G., Lazure C., Mbikay M., Chrétien M. Cloning and primary sequence of a mouse candidate prohormone convertase PC1 homologous to PC2, Furin, and Kex2: distinct chromosomal localization and messenger RNA distribution in brain and pituitary compared to PC2. Mol Endocrinol. 1991 Jan;5(1):111–122. doi: 10.1210/mend-5-1-111. [DOI] [PubMed] [Google Scholar]
- Smeekens S. P., Avruch A. S., LaMendola J., Chan S. J., Steiner D. F. Identification of a cDNA encoding a second putative prohormone convertase related to PC2 in AtT20 cells and islets of Langerhans. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):340–344. doi: 10.1073/pnas.88.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S. P., Steiner D. F. Identification of a human insulinoma cDNA encoding a novel mammalian protein structurally related to the yeast dibasic processing protease Kex2. J Biol Chem. 1990 Feb 25;265(6):2997–3000. [PubMed] [Google Scholar]
- Stieneke-Gröber A., Vey M., Angliker H., Shaw E., Thomas G., Roberts C., Klenk H. D., Garten W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. EMBO J. 1992 Jul;11(7):2407–2414. doi: 10.1002/j.1460-2075.1992.tb05305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Harada A., Hayashi Y., Wada K., Asaka J., Gotoh B., Ogasawara T., Nagai Y. Primary structure of the virus activating protease from chick embryo. Its identity with the blood clotting factor Xa. FEBS Lett. 1991 Jun 3;283(2):281–285. doi: 10.1016/0014-5793(91)80608-6. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thorne B. A., Thomas L., Allen R. G., Hruby D. E., Fuller R., Thorner J. Yeast KEX2 endopeptidase correctly cleaves a neuroendocrine prohormone in mammalian cells. Science. 1988 Jul 8;241(4862):226–230. doi: 10.1126/science.3291117. [DOI] [PubMed] [Google Scholar]
- Thomas L., Leduc R., Thorne B. A., Smeekens S. P., Steiner D. F., Thomas G. Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda T., Gotoh B., Sakaguchi T., Kida H., Nagai Y. Identification of amino acids relevant to three antigenic determinants on the fusion protein of Newcastle disease virus that are involved in fusion inhibition and neutralization. J Virol. 1988 Nov;62(11):4427–4430. doi: 10.1128/jvi.62.11.4427-4430.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi T., Hirota H., Gotoh B., Kuma K., Miyata T., Nagai Y. Newcastle disease virus evolution. II. Lack of gene recombination in generating virulent and avirulent strains. Virology. 1989 Apr;169(2):273–282. doi: 10.1016/0042-6822(89)90152-9. [DOI] [PubMed] [Google Scholar]
- Toyoda T., Sakaguchi T., Imai K., Inocencio N. M., Gotoh B., Hamaguchi M., Nagai Y. Structural comparison of the cleavage-activation site of the fusion glycoprotein between virulent and avirulent strains of Newcastle disease virus. Virology. 1987 May;158(1):242–247. doi: 10.1016/0042-6822(87)90261-3. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Nakagawa T., Ikemizu J., Nagahama M., Murakami K., Nakayama K. Sequence requirements for precursor cleavage within the constitutive secretory pathway. J Biol Chem. 1992 Apr 25;267(12):8270–8274. [PubMed] [Google Scholar]
- Webster R. G., Rott R. Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell. 1987 Aug 28;50(5):665–666. doi: 10.1016/0092-8674(87)90321-7. [DOI] [PubMed] [Google Scholar]
- Wise R. J., Barr P. J., Wong P. A., Kiefer M. C., Brake A. J., Kaufman R. J. Expression of a human proprotein processing enzyme: correct cleavage of the von Willebrand factor precursor at a paired basic amino acid site. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9378–9382. doi: 10.1073/pnas.87.23.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Nakayama Y., Nagura H., Toyoda T., Nishikawa K., Hamaguchi M., Nagai Y. Inhibition of the assembly of Newcastle disease virus by monensin. Virus Res. 1986 Feb;4(2):179–195. doi: 10.1016/0168-1702(86)90040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasa Y., Paul J. I., Whittaker J., Steiner D. F. Effects of amino acid replacements within the tetrabasic cleavage site on the processing of the human insulin receptor precursor expressed in Chinese hamster ovary cells. J Biol Chem. 1990 Oct 5;265(28):17230–17237. [PubMed] [Google Scholar]
- van de Ven W. J., Voorberg J., Fontijn R., Pannekoek H., van den Ouweland A. M., van Duijnhoven H. L., Roebroek A. J., Siezen R. J. Furin is a subtilisin-like proprotein processing enzyme in higher eukaryotes. Mol Biol Rep. 1990 Nov;14(4):265–275. doi: 10.1007/BF00429896. [DOI] [PubMed] [Google Scholar]
- van den Ouweland A. M., van Duijnhoven H. L., Keizer G. D., Dorssers L. C., Van de Ven W. J. Structural homology between the human fur gene product and the subtilisin-like protease encoded by yeast KEX2. Nucleic Acids Res. 1990 Feb 11;18(3):664–664. doi: 10.1093/nar/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]