Abstract
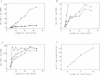
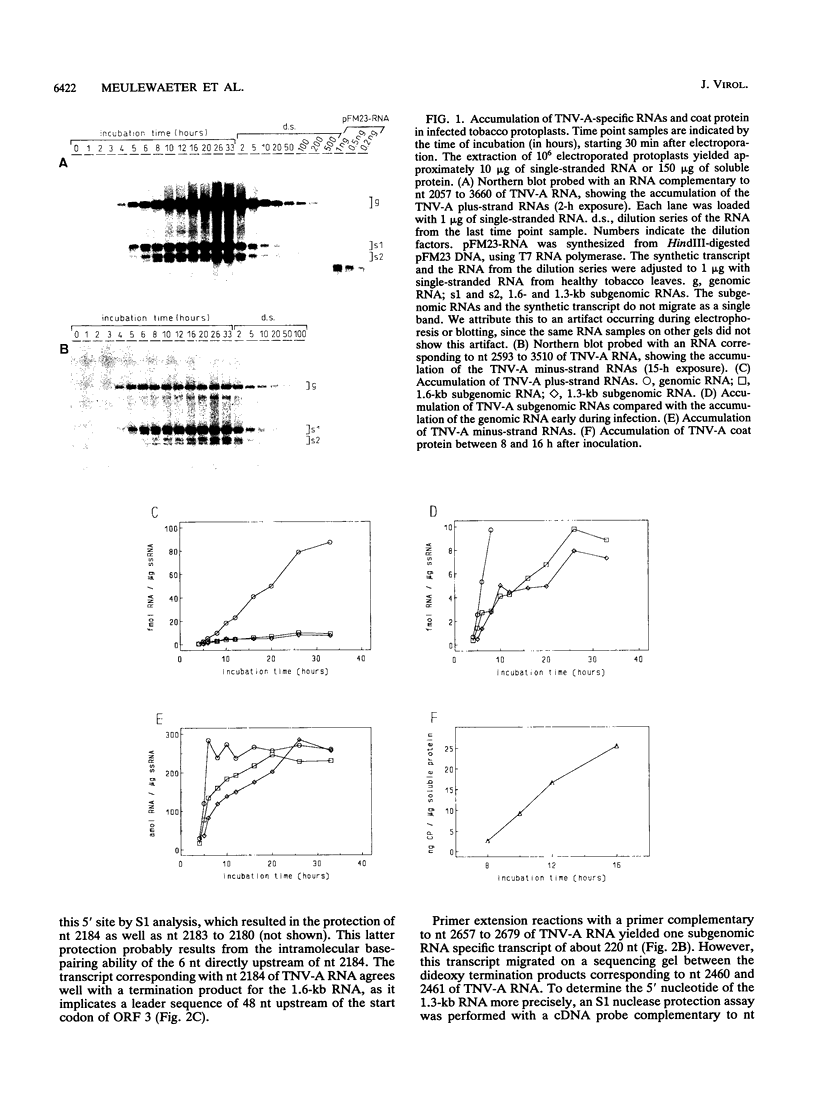
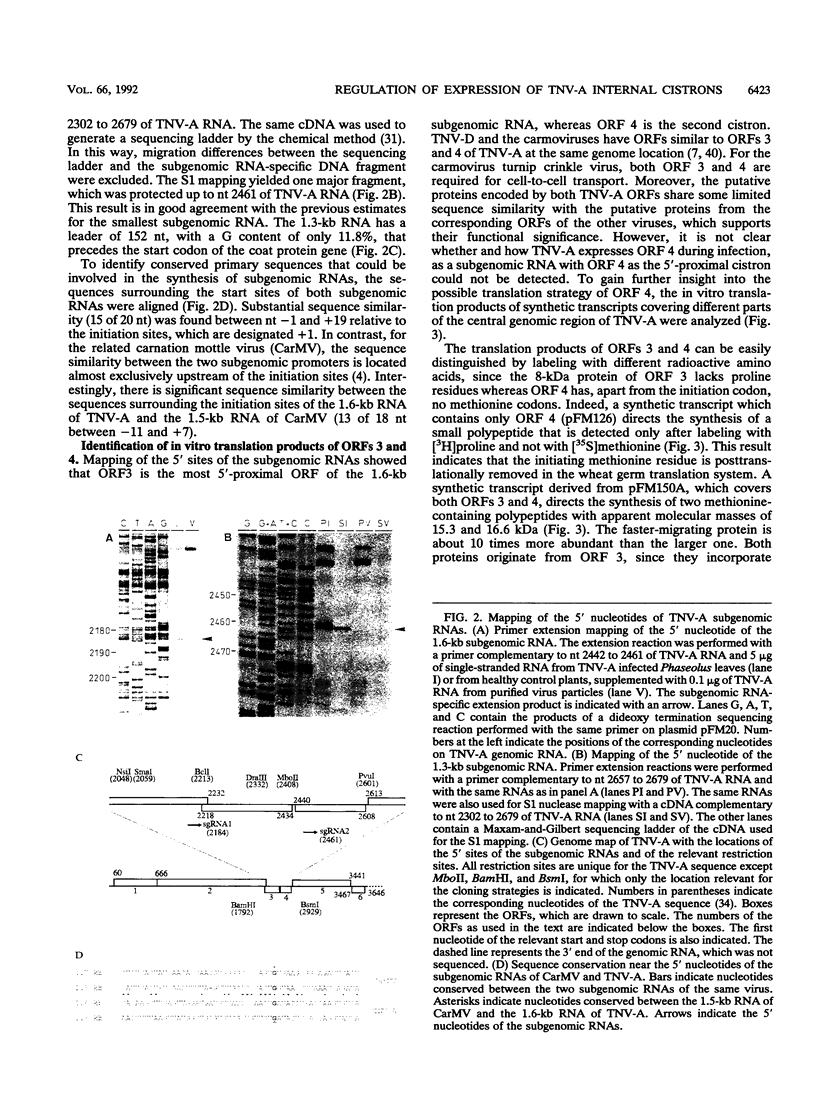
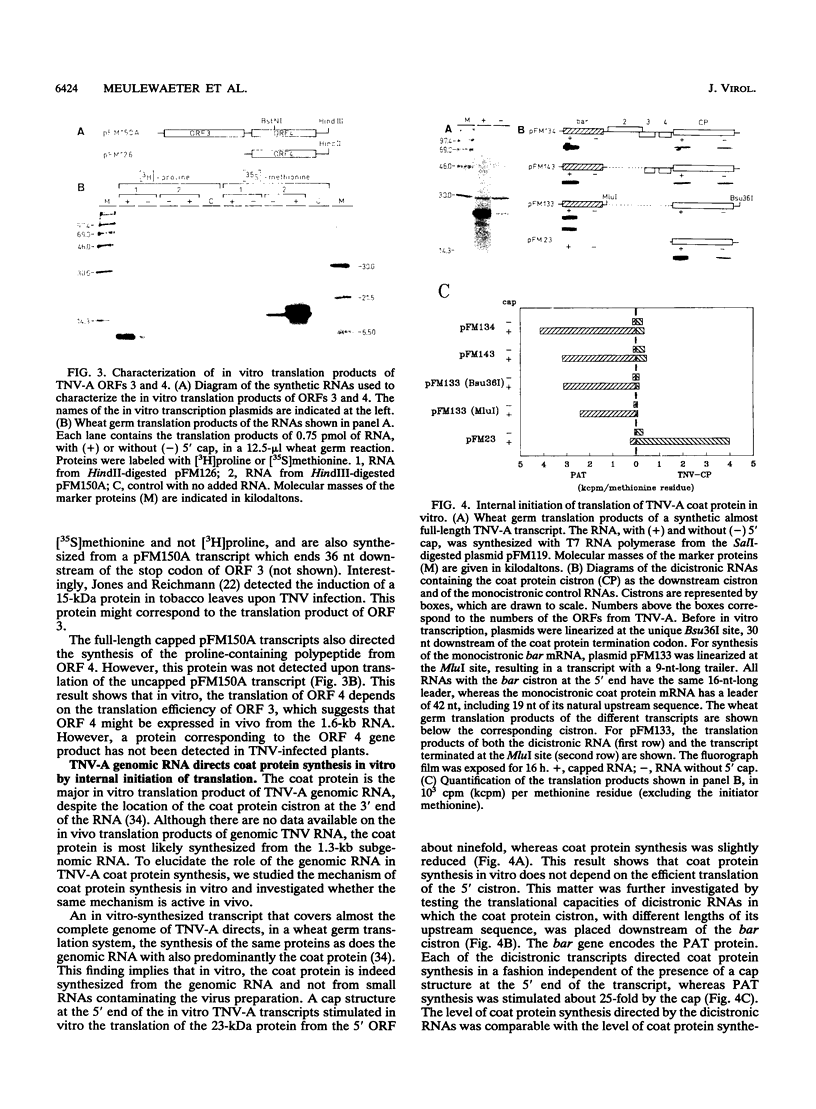
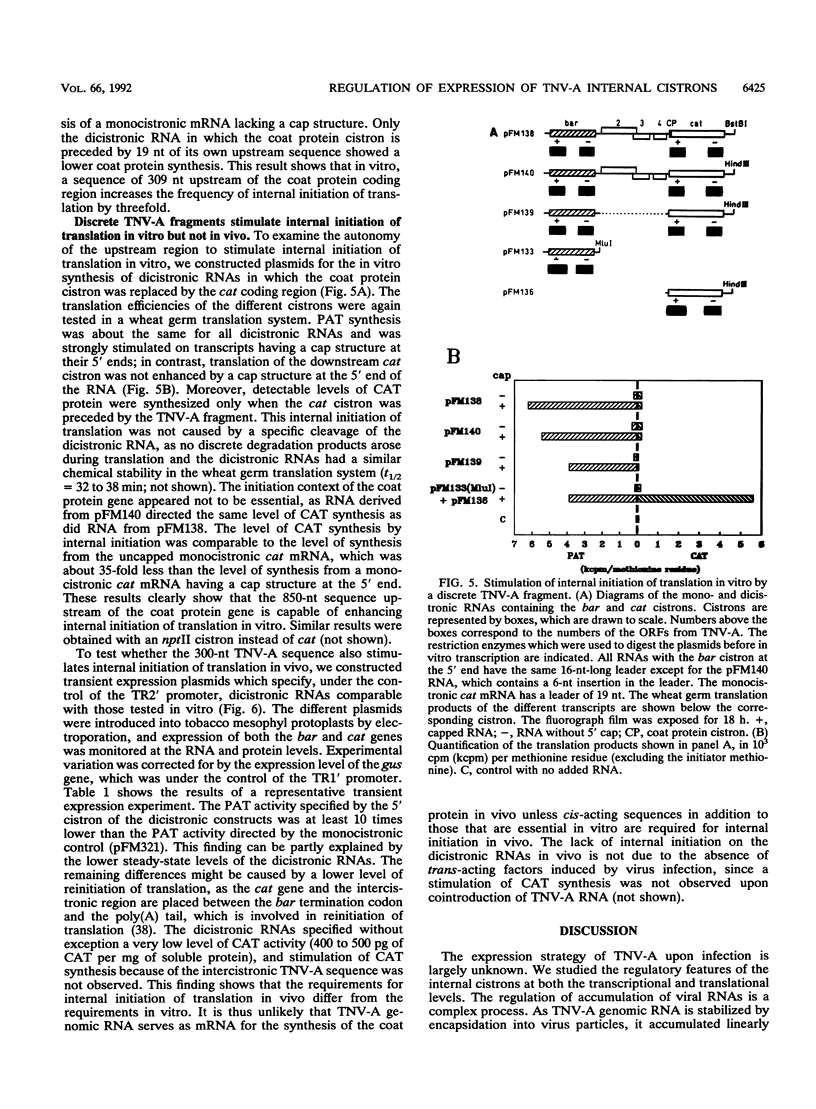
Upon infection of tobacco protoplasts, the genomic RNA of tobacco necrosis virus strain A (TNV-A) accumulates linearly in time. The accumulation patterns of the two subgenomic RNAs resemble those of endogenous mRNAs in that the peak levels are reached after several hours. The accumulation of the 1.3-kb subgenomic RNA is delayed by 1 h compared with that of the 1.6-kb subgenomic RNA, which illustrates the important role of the subgenomic RNAs in the regulation of TNV-A gene expression. The locations of the 5' nucleotides of the subgenomic RNAs reveal that the 5'-proximal cistrons of the 1.6- and 1.3-kb RNAs encode an 8-kDa protein from open reading frame (ORF) 3 and the coat protein from ORF 5, respectively. In a wheat germ translation system, a synthetic transcript resembling the 1.6-kb RNA expresses both ORFs 3 and 4. Moreover, the synthesis of the 6-kDa protein from ORF 4 depends on the translation efficiency of ORF 3, suggesting that in vivo, ORFs 3 and 4 are both expressed from the 1.6-kb RNA. The major in vitro translation product of TNV-A genomic RNA is the coat protein. We show that the region upstream of the coat protein promotes internal initiation of translation in vitro. However, this region is functionally inactive in vivo, suggesting that TNV-A genomic RNA is not important for coat protein synthesis in plants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angenon G., Uotila J., Kurkela S. A., Teeri T. H., Botterman J., Van Montagu M., Depicker A. Expression of dicistronic transcriptional units in transgenic tobacco. Mol Cell Biol. 1989 Dec;9(12):5676–5684. doi: 10.1128/mcb.9.12.5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. D., Botterman J., Vandewiele M., Dockx J., Thoen C., Gosselé V., Movva N. R., Thompson C., Montagu M. V., Leemans J. Engineering herbicide resistance in plants by expression of a detoxifying enzyme. EMBO J. 1987 Sep;6(9):2513–2518. doi: 10.1002/j.1460-2075.1987.tb02537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone F. J., Britten R. J., Davidson E. H. Mapping of gene transcripts by nuclease protection assays and cDNA primer extension. Methods Enzymol. 1987;152:611–632. doi: 10.1016/0076-6879(87)52069-9. [DOI] [PubMed] [Google Scholar]
- Citovsky V., Zambryski P. How do plant virus nucleic acids move through intercellular connections? Bioessays. 1991 Aug;13(8):373–379. doi: 10.1002/bies.950130802. [DOI] [PubMed] [Google Scholar]
- Cornelissen M., Vandewiele M. Both RNA level and translation efficiency are reduced by anti-sense RNA in transgenic tobacco. Nucleic Acids Res. 1989 Feb 11;17(3):833–843. doi: 10.1093/nar/17.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts R. H., Rigden J. E., Slabas A. R., Lomonossoff G. P., Wise P. J. The complete nucleotide sequence of tobacco necrosis virus strain D. J Gen Virol. 1991 Jul;72(Pt 7):1521–1529. doi: 10.1099/0022-1317-72-7-1521. [DOI] [PubMed] [Google Scholar]
- De Greve H., Dhaese P., Seurinck J., Lemmers M., Van Montagu M., Schell J. Nucleotide sequence and transcript map of the Agrobacterium tumefaciens Ti plasmid-encoded octopine synthase gene. J Mol Appl Genet. 1982;1(6):499–511. [PubMed] [Google Scholar]
- French R., Ahlquist P. Characterization and engineering of sequences controlling in vivo synthesis of brome mosaic virus subgenomic RNA. J Virol. 1988 Jul;62(7):2411–2420. doi: 10.1128/jvi.62.7.2411-2420.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fütterer J., Hohn T. Translation of a polycistronic mRNA in the presence of the cauliflower mosaic virus transactivator protein. EMBO J. 1991 Dec;10(12):3887–3896. doi: 10.1002/j.1460-2075.1991.tb04958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Lucas W. J., Walbot V. Visualizing mRNA expression in plant protoplasts: factors influencing efficient mRNA uptake and translation. Plant Cell. 1989 Mar;1(3):301–311. doi: 10.1105/tpc.1.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargouri R., Joshi R. L., Bol J. F., Astier-Manifacier S., Haenni A. L. Mechanism of synthesis of turnip yellow mosaic virus coat protein subgenomic RNA in vivo. Virology. 1989 Aug;171(2):386–393. doi: 10.1016/0042-6822(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker D. L., Petty I. T., Wei N., Morris T. J. Turnip crinkle virus genes required for RNA replication and virus movement. Virology. 1992 Jan;186(1):1–8. doi: 10.1016/0042-6822(92)90055-t. [DOI] [PubMed] [Google Scholar]
- Hershey J. W. Translational control in mammalian cells. Annu Rev Biochem. 1991;60:717–755. doi: 10.1146/annurev.bi.60.070191.003441. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Ohno T., Okada Y. Specific cessation of minus-strand RNA accumulation at an early stage of tobacco mosaic virus infection. J Virol. 1991 Feb;65(2):861–868. doi: 10.1128/jvi.65.2.861-868.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones I. M., Reichmann M. E. The proteins synthesized in tobacco leaves infected with tobacco necrosis virus and satellite tobacco necrosis virus. Virology. 1973 Mar;52(1):49–56. doi: 10.1016/0042-6822(73)90397-8. [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol Cell Biol. 1989 Nov;9(11):5073–5080. doi: 10.1128/mcb.9.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehto K., Grantham G. L., Dawson W. O. Insertion of sequences containing the coat protein subgenomic RNA promoter and leader in front of the tobacco mosaic virus 30K ORF delays its expression and causes defective cell-to-cell movement. Virology. 1990 Jan;174(1):145–157. doi: 10.1016/0042-6822(90)90063-w. [DOI] [PubMed] [Google Scholar]
- Lesnaw J. A., Reichmann M. E. The structure of tobacco necrosis virus. I. The protein subunit and the nature of the nucleic acid. Virology. 1969 Dec;39(4):729–737. doi: 10.1016/0042-6822(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Marsh L. E., Huntley C. C., Pogue G. P., Connell J. P., Hall T. C. Regulation of (+):(-)-strand asymmetry in replication of brome mosaic virus RNA. Virology. 1991 May;182(1):76–83. doi: 10.1016/0042-6822(91)90650-z. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Gavis E. R., Kingsbury R., Axel R. Analysis of transcriptional regulatory signals of the HSV thymidine kinase gene: identification of an upstream control region. Cell. 1981 Aug;25(2):385–398. doi: 10.1016/0092-8674(81)90057-x. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulewaeter F., Seurinck J., Van Emmelo J. Genome structure of tobacco necrosis virus strain A. Virology. 1990 Aug;177(2):699–709. doi: 10.1016/0042-6822(90)90536-z. [DOI] [PubMed] [Google Scholar]
- Miller W. A., Dreher T. W., Hall T. C. Synthesis of brome mosaic virus subgenomic RNA in vitro by internal initiation on (-)-sense genomic RNA. Nature. 1985 Jan 3;313(5997):68–70. doi: 10.1038/313068a0. [DOI] [PubMed] [Google Scholar]
- Munroe D., Jacobson A. Tales of poly(A): a review. Gene. 1990 Jul 16;91(2):151–158. doi: 10.1016/0378-1119(90)90082-3. [DOI] [PubMed] [Google Scholar]
- Riviere C. J., Rochon D. M. Nucleotide sequence and genomic organization of melon necrotic spot virus. J Gen Virol. 1990 Sep;71(Pt 9):1887–1896. doi: 10.1099/0022-1317-71-9-1887. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kuyl A. C., Neeleman L., Bol J. F. Role of alfalfa mosaic virus coat protein in regulation of the balance between viral plus and minus strand RNA synthesis. Virology. 1991 Nov;185(1):496–499. doi: 10.1016/0042-6822(91)90807-n. [DOI] [PubMed] [Google Scholar]
- Velten J., Schell J. Selection-expression plasmid vectors for use in genetic transformation of higher plants. Nucleic Acids Res. 1985 Oct 11;13(19):6981–6998. doi: 10.1093/nar/13.19.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velten J., Velten L., Hain R., Schell J. Isolation of a dual plant promoter fragment from the Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1984 Dec 1;3(12):2723–2730. doi: 10.1002/j.1460-2075.1984.tb02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. A., Mackie G. A. Control and expression of 3' open reading frames in clover yellow mosaic virus. Virology. 1990 Dec;179(2):576–584. doi: 10.1016/0042-6822(90)90124-a. [DOI] [PubMed] [Google Scholar]
- van der Kuyl A. C., Langereis K., Houwing C. J., Jaspars E. M., Bol J. F. cis-acting elements involved in replication of alfalfa mosaic virus RNAs in vitro. Virology. 1990 Jun;176(2):346–354. doi: 10.1016/0042-6822(90)90004-b. [DOI] [PubMed] [Google Scholar]
- van der Kuyl A. C., Neeleman L., Bol J. F. Deletion analysis of cis- and trans-acting elements involved in replication of alfalfa mosaic virus RNA 3 in vivo. Virology. 1991 Aug;183(2):687–694. doi: 10.1016/0042-6822(91)90997-p. [DOI] [PubMed] [Google Scholar]