Abstract
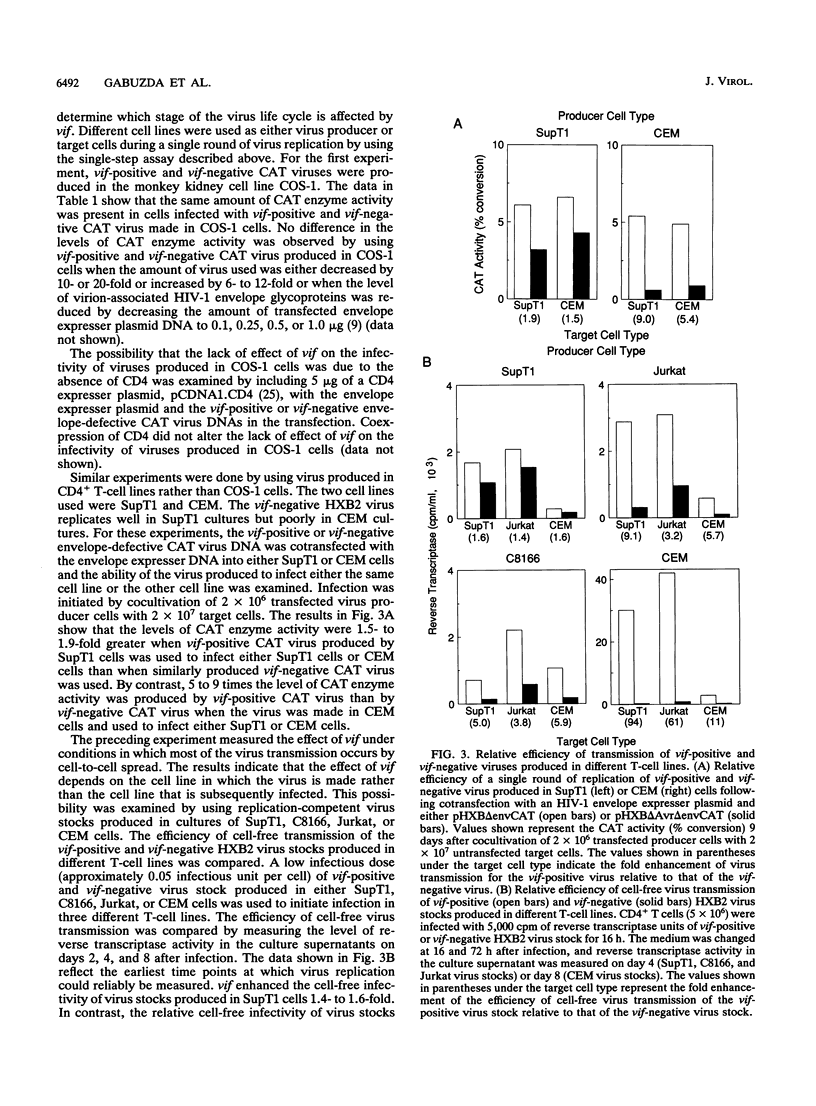
The viral infectivity factor gene vif of human immunodeficiency virus type 1 has been shown to affect the infectivity but not the production of virus particles. In this study, the effect of vif in the context of the HXB2 virus on virus replication in several CD4+ T-cell lines was investigated. vif was found to be required for replication in the CD4+ T-cell lines CEM and H9 as well as in peripheral blood T lymphocytes. vif was not required for replication in the SupT1, C8166, and Jurkat T-cell lines. The infectivity of vif-defective viruses depended on the cell type in which the virus was produced. In CEM cells, vif was required for production of virus capable of initiating infection in all cell lines studied. vif-defective virus produced by SupT1, C8166, and Jurkat cells and the monkey cell line COS-1 could initiate infection in multiple cell lines, including CEM and H9. These results suggest that vif can compensate for cellular factors required for production of infectious virus particles that are present in some cell lines such as SupT1, C8166, and Jurkat but are absent in others such as CEM and H9 as well as peripheral blood T lymphocytes. The effect of vif was not altered by deletion of the carboxyl terminus of gp41, a proposed target for vif (B. Guy, M. Geist, K. Dott, D. Spehner, M.-P. Kieny, and J.-P. Lecocq, J. Virol. 65:1325-1331, 1991). These studies demonstrate that vif enhances viral infectivity during virus production and also suggest that vif is likely to be important for natural infections.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akari H., Sakuragi J., Takebe Y., Tomonaga K., Kawamura M., Fukasawa M., Miura T., Shinjo T., Hayami M. Biological characterization of human immunodeficiency virus type 1 and type 2 mutants in human peripheral blood mononuclear cells. Arch Virol. 1992;123(1-2):157–167. doi: 10.1007/BF01317146. [DOI] [PubMed] [Google Scholar]
- Arya S. K., Gallo R. C. Three novel genes of human T-lymphotropic virus type III: immune reactivity of their products with sera from acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2209–2213. doi: 10.1073/pnas.83.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Dedera D., Hu W., Vander Heyden N., Ratner L. Viral protein R of human immunodeficiency virus types 1 and 2 is dispensable for replication and cytopathogenicity in lymphoid cells. J Virol. 1989 Jul;63(7):3205–3208. doi: 10.1128/jvi.63.7.3205-3208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmrich F., Horneff G., Becker W., Lüke W., Potocnik A., Kanzy U., Kalden J. R., Burmester G. An anti-CD4 antibody for treatment of chronic inflammatory arthritis. Agents Actions Suppl. 1991;32:165–170. doi: 10.1007/978-3-0348-7405-2_22. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Ivanoff L., Chamberlain M., Petteway S., Ratner L., Gallo R. C., Wong-Staal F. The sor gene of HIV-1 is required for efficient virus transmission in vitro. Science. 1987 Aug 21;237(4817):888–893. doi: 10.1126/science.3497453. [DOI] [PubMed] [Google Scholar]
- Gabuzda D. H., Lever A., Terwilliger E., Sodroski J. Effects of deletions in the cytoplasmic domain on biological functions of human immunodeficiency virus type 1 envelope glycoproteins. J Virol. 1992 Jun;66(6):3306–3315. doi: 10.1128/jvi.66.6.3306-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett E. D., Tiley L. S., Cullen B. R. Rev activates expression of the human immunodeficiency virus type 1 vif and vpr gene products. J Virol. 1991 Mar;65(3):1653–1657. doi: 10.1128/jvi.65.3.1653-1657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy B., Geist M., Dott K., Spehner D., Kieny M. P., Lecocq J. P. A specific inhibitor of cysteine proteases impairs a Vif-dependent modification of human immunodeficiency virus type 1 Env protein. J Virol. 1991 Mar;65(3):1325–1331. doi: 10.1128/jvi.65.3.1325-1331.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth E., Kowalski M., Gabuzda D., Olshevsky U., Haseltine W., Sodroski J. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J Virol. 1990 May;64(5):2416–2420. doi: 10.1128/jvi.64.5.2416-2420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan N. C., Franchini G., Wong-Staal F., DuBois G. C., Robey W. G., Lautenberger J. A., Papas T. S. Identification of HTLV-III/LAV sor gene product and detection of antibodies in human sera. Science. 1986 Mar 28;231(4745):1553–1555. doi: 10.1126/science.3006245. [DOI] [PubMed] [Google Scholar]
- Langhoff E., Terwilliger E. F., Bos H. J., Kalland K. H., Poznansky M. C., Bacon O. M., Haseltine W. A. Replication of human immunodeficiency virus type 1 in primary dendritic cell cultures. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7998–8002. doi: 10.1073/pnas.88.18.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. H., Coligan J. E., Allan J. S., McLane M. F., Groopman J. E., Essex M. A new HTLV-III/LAV protein encoded by a gene found in cytopathic retroviruses. Science. 1986 Mar 28;231(4745):1546–1549. doi: 10.1126/science.3006243. [DOI] [PubMed] [Google Scholar]
- Luciw P. A., Cheng-Mayer C., Levy J. A. Mutational analysis of the human immunodeficiency virus: the orf-B region down-regulates virus replication. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1434–1438. doi: 10.1073/pnas.84.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Queen C., Baltimore D. Immunoglobulin gene transcription is activated by downstream sequence elements. Cell. 1983 Jul;33(3):741–748. doi: 10.1016/0092-8674(83)90016-8. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rho H. M., Poiesz B., Ruscetti F. W., Gallo R. C. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology. 1981 Jul 15;112(1):355–360. doi: 10.1016/0042-6822(81)90642-5. [DOI] [PubMed] [Google Scholar]
- Sakai K., Ma X. Y., Gordienko I., Volsky D. J. Recombinational analysis of a natural noncytopathic human immunodeficiency virus type 1 (HIV-1) isolate: role of the vif gene in HIV-1 infection kinetics and cytopathicity. J Virol. 1991 Nov;65(11):5765–5773. doi: 10.1128/jvi.65.11.5765-5773.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Pavlakis G. N. Expression of human immunodeficiency virus type 1 vif and vpr mRNAs is Rev-dependent and regulated by splicing. Virology. 1991 Aug;183(2):677–686. doi: 10.1016/0042-6822(91)90996-o. [DOI] [PubMed] [Google Scholar]
- Shin J., Dunbrack R. L., Jr, Lee S., Strominger J. L. Signals for retention of transmembrane proteins in the endoplasmic reticulum studied with CD4 truncation mutants. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1918–1922. doi: 10.1073/pnas.88.5.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. D., Shatsky M., Cohen P. S., Warnke R., Link M. P., Glader B. E. Monoclonal antibody and enzymatic profiles of human malignant T-lymphoid cells and derived cell lines. Cancer Res. 1984 Dec;44(12 Pt 1):5657–5660. [PubMed] [Google Scholar]
- Sodroski J., Goh W. C., Rosen C., Tartar A., Portetelle D., Burny A., Haseltine W. Replicative and cytopathic potential of HTLV-III/LAV with sor gene deletions. Science. 1986 Mar 28;231(4745):1549–1553. doi: 10.1126/science.3006244. [DOI] [PubMed] [Google Scholar]
- Sonigo P., Alizon M., Staskus K., Klatzmann D., Cole S., Danos O., Retzel E., Tiollais P., Haase A., Wain-Hobson S. Nucleotide sequence of the visna lentivirus: relationship to the AIDS virus. Cell. 1985 Aug;42(1):369–382. doi: 10.1016/s0092-8674(85)80132-x. [DOI] [PubMed] [Google Scholar]
- Strebel K., Daugherty D., Clouse K., Cohen D., Folks T., Martin M. A. The HIV 'A' (sor) gene product is essential for virus infectivity. Nature. 1987 Aug 20;328(6132):728–730. doi: 10.1038/328728a0. [DOI] [PubMed] [Google Scholar]
- Talbott R. L., Sparger E. E., Lovelace K. M., Fitch W. M., Pedersen N. C., Luciw P. A., Elder J. H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger E. F., Godin B., Sodroski J. G., Haseltine W. A. Construction and use of a replication-competent human immunodeficiency virus (HIV-1) that expresses the chloramphenicol acetyltransferase enzyme. Proc Natl Acad Sci U S A. 1989 May;86(10):3857–3861. doi: 10.1073/pnas.86.10.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele B., Braig H. R., Ehm I., Kunze R., Ruf B. Influence of sulfated carbohydrates on the accessibility of CD4 and other CD molecules on the cell surface and implications for human immunodeficiency virus infection. Eur J Immunol. 1989 Jun;19(6):1161–1164. doi: 10.1002/eji.1830190630. [DOI] [PubMed] [Google Scholar]
- Weiss A., Wiskocil R. L., Stobo J. D. The role of T3 surface molecules in the activation of human T cells: a two-stimulus requirement for IL 2 production reflects events occurring at a pre-translational level. J Immunol. 1984 Jul;133(1):123–128. [PubMed] [Google Scholar]




