Abstract
The major subassemblies of virulence-associated P pili, the pilus rod (comprised of PapA) and tip fibrillum (comprised of PapE), were reconstituted from purified chaperone-subunit complexes in vitro. Subunits are held in assembly-competent conformations in chaperone-subunit complexes prior to their assembly into mature pili. The PapD chaperone binds, in part, to a conserved motif present at the C terminus of the subunits via a beta zippering interaction. Amino acid residues in this conserved motif were also found to be essential for subunit–subunit interactions necessary for the formation of pili, thus revealing a molecular mechanism whereby the PapD chaperone may prevent premature subunit–subunit interactions in the periplasm. Uncapping of the chaperone-protected C terminus of PapA and PapE was mimicked in vitro by freeze–thaw techniques and resulted in the formation of pilus rods and tip fibrillae, respectively. A mutation in the leading edge of the beta zipper of PapA produces pilus rods with an altered helical symmetry and azimuthal disorder. This change in the number of subunits per turn of the helix most likely reflects involvement of the leading edge of the beta zipper in forming a right-handed helical cylinder. Organelle development is a fundamental process in all living cells, and these studies shed new light on how immunoglobulin-like chaperones govern the formation of virulence-associated organelles in pathogenic bacteria.
The development of more than 25 organelles of attachment in pathogenic bacteria proceeds via the chaperone/usher-dependent pathway (1). This pathway uses a periplasmic chaperone that interacts with monomer subunits, protecting them from aggregation and proteolysis by forming chaperone–subunit complexes in the periplasm. The complexes are targeted to outer membrane assembly sites comprised of proteins termed ushers. At this site, the chaperone is dissociated and the subunit is ushered across the outer membrane into the pilus assembly (1). Uropathogenic strains of Escherichia coli associated with acute pyelonephritis often express P pili, which are considered the prototype organelles assembled by the chaperone/usher pathway. Genes important in pilus biogenesis, papA–K, are linked in an operon, and their expression is coordinately controlled (2). P pili are comprised of six distinct structural proteins that interact to form a composite fiber consisting of two distinct subassemblies (3): (i) a 68-Å-diameter pilus rod comprised of PapA subunits arranged in a right-handed helical cylinder having 3.28 subunits per turn (u/t) (4) and (ii) a thin fibrillum (≈20 Å diameter) comprised mostly of repeating PapE subunits arranged in an open helical configuration (3). The pilus rod has a 15-Å helical cavity winding about the helical axis and communicating with the external environment by a set of radial channels (5). PapA and PapE are the only two pilus subunits known to self-associate into oligomeric subassemblies in vivo. The two subassemblies are joined together by the PapK adapter protein (6), and the pilus rod is anchored in the outer membrane by the PapH protein (2). The PapG adhesin, a virulence factor in uropathogenic E. coli (7), is joined to the distal end of the tip fibrillum via the PapF adapter protein (3, 6). PapG, PapF, and PapK do not form homo-oligomeric subassemblies in vivo (8).
The assembly of P pili requires the PapD chaperone, which has served as a prototype chaperone for the chaperone/usher pathway. The three-dimensional structure of PapD, solved by Holmgren and Brändén (9), consists of two globular domains oriented as in a boomerang. Each domain is a beta-barrel structure formed by two antiparallel beta pleated sheets that have a topology similar to an immunoglobulin fold (9). The PapD chaperone binds each subunit type, preventing their premature association in the periplasm by forming chaperone–subunit complexes (10, 11). When bound to PapD, subunits are “capped” and do not participate in subunit–subunit interactions, with the exception of PapA, which can form dimers (DA2) and possibly trimers (DA3) and tetramers (DA4) in association with PapD (8, 11). Chaperone–subunit complexes are targeted to the outer membrane PapC usher where chaperone dissociation is coupled with incorporation of the subunits into the growing organelles (12) (see Fig. 1 for a model of pilus assembly).
Figure 1.
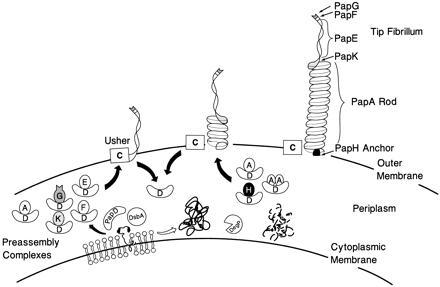
Model of pilus assembly. Details of the model are discussed in the text. PapD has an immunoglobulin-like three-dimensional structure (9) and is the prototype member of a large family of PapD-like chaperones required for pilus assembly in Gram-negative bacteria (13). DsbA mediates disulfide bond formation in PapD and in the pilus subunits (14). DsbA is required for the correct folding of PapD. Closed arrow depicts pathway subunits travel in the presence of PapD chaperone. Subunits misfold and are proteolytically degraded by the DegP protease in the absence of an interaction with PapD (open arrow) (C.H.J. and S.J.H., unpublished work). Chaperone–subunit complexes are targeted to the PapC usher protein (12) where pilus assembly occurs; an essential step in assembly is the displacement of PapD (uncapping). The PapD chaperone has been shown to interact with pilus subunits, in part, via the COOH terminal motif (15). Binding of PapD to an assembly surface on the subunits modulates the formation of the pilus rod.
The molecular basis of the PapD–PapG interaction was solved in part by cocrystallizing PapD with a peptide corresponding to the C-terminal 19 amino acids of PapG (15). The peptide bound in an extended conformation with its C terminus anchored in the cleft via hydrogen bonding to the invariant Arg-8 and Lys-112 residues that probably function as a molecular anchor in all of the PapD-like chaperones for binding pilus subunits. Site-directed mutations in Arg-8 and Lys-112 abolished the ability of PapD to bind subunits and mediate pilus assembly in vivo, indicating that the PapD-peptide crystal structure is a reflection of PapD–PapG interactions (15, 16). The positioning of the peptide along the exposed edge of PapD’s G1 β-strand was mostly the result of backbone hydrogen bonds forming a β-sheet structure between the chaperone and peptide, defined as a “beta zipper” (15). These data suggested a general model in which the C termini of newly translocated subunits zipper to the G1 β-strand of the chaperone. PapD may provide a platform for β-strand zippering allowing the subunits to be maintained in assembly-competent conformations.
Since chaperones are thought to cap and uncap the associative surfaces of pilus subunits (11), we investigated whether the subunit’s C terminus comprises part of the surface used in subunit–subunit associations after chaperone uncapping. We used a combination of molecular biology, biochemistry, and biophysics to investigate the role of the C terminus of subunits in pilus assembly using both in vivo and in vitro systems. Uncapping of the chaperone-protected PapA C terminus was mimicked in vitro by freeze–thaw techniques and led to the formation of pilus rod subassemblies, whereas the uncapping of PapE led to the formation of tip fibrillum subassemblies. An analysis of site-directed mutations in and around the beta zipper of PapA revealed the molecular details of an interactive surface on PapA required for the formation of a 7-nm right-handed helical rod.
MATERIALS AND METHODS
Bacterial Strains and Genetic Constructs.
E. coli strains HB101, KS474 (degP−), and the isogenic parent KS272 (17) were used as host strains for cloning, mutagenesis, expression, and purification of chaperone–subunit complexes. PCR mutagenesis was performed as described (18). Bacteria were grown to an A600 of 0.6 in Luria broth, at which time PapD was induced from the arabinose promoter in pHJ9203 (C.H.J. and S.J.H., unpublished data) using 0.05% l-arabinose, and PapA derivatives were induced from the Ptac promoter in pMMB66 derivative plasmids using 0.01 mM isopropyl β-d-thiogalactoside. Induction time was 60 min. Periplasm containing PapD and PapA was prepared as described (16). Mutant PapA proteins were found to be stabilized in the degP− background (C.H.J. and S.J.H., unpublished work).
Protein Purification and Analysis.
PapD–PapA complexes were purified to homogeneity as described (8) with the following modifications. Periplasm containing PapD–PapA complexes was dialyzed into bis·Tris (pH 6.35), and applied to a Mono Q (Pharmacia) anion exchange column. The PapD–PapA complexes appeared in the flow-through fraction with ≈70% purification. The PapD–PapA samples were then brought to 1 M (NH4)SO4, 50 mM phosphate buffer (pH 7.0), and applied to phenyl superose (Pharmacia) (8). Pharmacia 3–9 isoelectric focusing gels were run according to the manufacturer and silver stained using the Phast system (Pharmacia). PapA multimers were detected by SDS/PAGE following treatment of periplasmic fractions with SDS/PAGE loading buffer (1.5% SDS) for 5 min at 25°C or 95°C prior to application to SDS/PAGE (8). Western blot analysis was performed using polyclonal antipilus antibody raised in rabbit.
In Vitro Assembly/Electron Microscopy.
Samples were quick frozen on dry ice for at least 3 min and allowed to thaw at 25°C for at least 5 min. Following freeze–thaw, a sample (5 μl of 0.05 mg/ml chaperone–subunit complex; PapD–PapK was used at 1 mg/ml) was applied to 300-mesh carbon coated grids. After allowing ≈5 min for adsorption, grids were washed with 0.5 mM Tris (pH 7.5) and negatively stained with 2% uranyl acetate. Specimens were examined and photographed using a Philips CM12 electron microscope operating at 100 kV (magnification ×75,000). Micrographs were recorded on Kodak SO-163 film. Helical symmetry (19) was calculated using a helical lattice of 23 subunits in seven turns of the helix (l = 7n + 23m) (5). Statistical significance was confirmed using the Student’s t test.
RESULTS
Reconstitution of Pilus Assembly in Vitro from Chaperone–Subunit Complexes.
PapD–subunit complexes that accumulate in the periplasmic space in the absence of the PapC usher (11, 12) were isolated and investigated. Freeze–thaw conditions that led to the dissociation of chaperone–subunit complexes and the formation of pilus rod-like subassemblies were established. High-resolution electron microscopy of a purified preparation of PapD–PapA complexes (a mixture of 1:1 PapD–PapA and 1:2 PapD–PapA complexes) prior to freeze–thaw revealed few if any visible oligomeric assemblies (Fig. 2A). Three freeze–thaw cycles of the PapD–PapA complexes resulted in the formation of short ring-like structures with the appearance expected of a single helical turn of the pilus rod (3–4 subunits) with an axial hole (Fig. 2B). Some short rods were also seen. After 10 freeze–thaw cycles, many more rod-like fibers were visible, with structures very similar, possibly identical to, native pili. These fibers were up to one-tenth the length of a typical pilus rod, forming subassemblies of ≈100 PapA subunits (Fig. 2 C and E). Freeze–thaw of PapD–PapA complexes in the presence of a 10-fold excess of PapD effectively blocked the formation of rod-like structures (Fig. 2D), suggesting that PapD binds competitively to a surface on PapA that, at least in part, participates in subunit–subunit interactions when in the free state. The incorporation of released PapA subunits into native-like pilus structures argues that subunit–subunit interactions in a pilus rod are energetically favored over chaperone–subunit interactions. In addition, the immediate formation of pilus subassemblies after chaperone uncapping demonstrates the function of the chaperone in regulating the development of the organelle.
Figure 2.
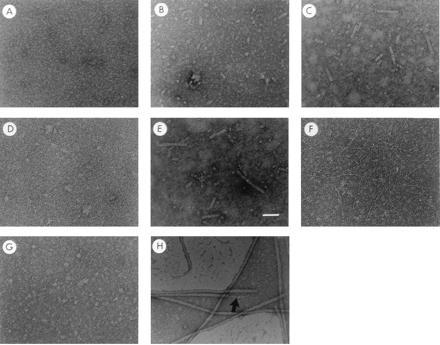
In vitro assembly of pilus-like or tip fibrillum-like fibers after chaperone uncapping. Panels A–H are electron micrographs of chaperone–subunit complexes following rapid freeze-thaw conditions. PapD–PapA complexes (a mixture of DA, 1:1 PapD–PapA, and DA2, 1:2 PapD–PapA) before (A) and after three (B) or 10 (C) freeze-thaw cycles. (D and E) The PapD–PapA complexes after 10 freeze-thaw cycles in the presence (D) or absence (E) of a 10-fold excess of free PapD. Freeze-thaw treatment of purified PapD-PapE complex and PapD-PapK complex is shown in F and G, respectively. H is an electron micrograph of purified P pili showing the composite architecture of the fiber: a tip fibrillum (→) joined end to end to a pilus rod. Magnification bar in E = 500 Å.
Chaperone–subunit interactions can be exchanged for subunit–subunit interactions in vitro only for subunits known to self-associate in vivo (8). PapD was coexpressed with PapE, and PapD–PapE complexes were partially purified from periplasmic extracts as described (12) and examined after freeze–thaw. Under these conditions, thin fibrillae-like subassemblies were observed that were structurally indistinguishable from the tip fibrillae present at the distal ends of pilus rods (Fig. 2F). These results indicate that the molecular architecture of the fiber is determined by the subunit type in the chaperone–subunit complex. PapD–PapK complexes were also purified and examined by electron microscopy. Even at twice the concentration of the DE or DA complexes, and after repeated freeze–thaw cycles, the PapK adapter protein did not participate in the formation of oligomeric subassemblies (Fig. 2G). This indicates that no strong PapK–PapK interactions were established in vitro, in contrast to the self-assembling subunits, PapA and PapE.
Effects of PapA Point Mutations on Pilus Formation.
Bullitt and Makowski (5) recently demonstrated that the pilus rod is a thread-like PapA fibrillar polymer packaged into a right-handed helical rod. Each structural subunit in the pilus makes at least two interactions that are best described as head-to-tail interactions, defining the thread-like PapA and PapE fibrillar polymers and turn–turn interactions defining the right-handed helical PapA polymer. The molecular basis of the two distinct interactions made by PapA subunits was investigated by studying the effects of point mutations in conserved residues present in the C terminus of pilus subunits. In all of the pilus subunits, a conserved glycine is positioned at the leading edge of the beta zipper, which in PapA corresponds to residue 150. Residues in or surrounding the beta zipper in PapA (residues 151–163 correspond to the known beta zipper of the PapG adhesin defined by cocrystallization studies) were mutagenized to investigate their function in the biogenesis of the rod organelle. Gly-150 of PapA was changed to alanine or threonine (G150A or G150T). Tyr-162 of PapA was changed to phenylalanine, as in PapG, or to leucine (Y162F or Y162L). Thr-148 and Glu-149, nonconserved residues, were mutagenized to valine and proline, respectively (T148V + E149P), making PapA identical to the PapK protein at these two positions. Alanine mutations were also constructed in Gly-15 and Gly-144, which are not part of the β-strand zipper but are conserved in all six PapA serotypes (20). Complementation of papA knockout mutations with each of the point mutant PapAs described above revealed that the G150T and Y162L mutants abolished formation of pilus rod subassemblies in vivo (data not shown). The G150A mutant reduced the number of pilus rods formed by about 40% and the Y162F mutation also caused a slight reduction in the total number of pili per bacterium. The T148V + E149P, G15A, and G144A mutants had no detectable effect on piliation (data not shown). The molecular basis of the effect of the mutations in G150 and Y162 on interactions that are critical in the development of pilus organelles was investigated using several assays (described below).
Dependence of Chaperone–Subunit Complex Formation on the Beta Zipper.
PapD binds to PapA to form two complexes: DA (1:1, PapD–PapA complex) and DA2 (1:2, PapD–PapA complex) (8). The effect of each mutation on DA and DA2 complex formation was investigated after coexpression of PapD with wild-type or mutant PapA proteins and preparation of periplasmic extracts. The degP strain KS474 was used as a host for expression of the PapA mutants, because the mutant subunits were more stable in the protease-deficient background. PapD, DA, and DA2 complexes migrate to well-characterized positions on isoelectric focusing gels (8, 12). When PapA was expressed in the absence of PapD, a free PapA band was not detected in the periplasmic extracts (Fig. 3A, lane 1). Efficient localization of PapA to the periplasmic space was dependent on PapD; moreover, aggregation of PapA was seen when PapA was expressed in the absence of the chaperone (C.H.J. and S.J.H., unpublished work) (Fig. 3B). PapD bound to and formed stable periplasmic DA complexes with wild-type PapA as well as with G150A (alanine substitution at Gly-150) or G150T (threonine substitution at Gly- 150) PapA as determined by silver stained isoelectric focusing gels (Fig. 3A, lanes 2, 5, and 7, respectively). Compared with the wild-type PapA control (Fig. 3A, lane 6), the G150T mutation significantly reduced DA2 complex formation (Fig. 3A, lane 7), arguing that the G150T mutation destabilized the PapA–PapA interaction in the complex, whereas the G150A mutant still formed the DA2 complex. The stable complex(es) that PapD formed with G150A PapA were purified by anion exchange and hydrophobic interaction chromatography (8) and were shown to support the formation of short rod subassemblies after freeze–thaw, although less efficiently than wild-type DA complexes (data not shown). Interestingly, Y162F and Y162L reduced or abolished, respectively, the formation of the DA complex but had little or no effect on the formation of the DA2 complex (Fig. 3A, lanes 3 and 4). This effect was opposite from that seen with the G150T mutation. Mutating the penultimate tyrosine to leucine most likely disrupts the ability of PapA to form the beta zippering interaction necessary for stable DA complex formation. PapD has been shown to have at least two separable interactive surfaces (21). A second PapA subunit in the DA2 complex may serve as a bridge to connect the first PapA (by binding to a surface on PapA that is distinct from the C terminus) to a second PapD site that is distinct from the G1 β-strand of PapD, thus stabilizing the interactions in the ternary complex.
Figure 3.

Point mutations in the Beta zipper differentially effect chaperone–subunit complex formation and subunit–subunit interactions. (A) Isoelectric focusing gel electrophoresis demonstrating DA (1:1) and DA2 (1:2) complexes. Note that substitution of Tyr-162 diminishes (Y162F, lane 3) or abolishes (Y162L, lane 4) the formation of the DA complex while having no effect on the DA2 complex. In contrast the G150T substitution greatly decreases the DA2 band while having no effect on the DA complex (lane 7). Note that the samples in lanes 1–5 were loaded from the top comb position while the samples in lanes 6 and 7 were loaded from the bottom comb position, accounting for a somewhat cleaner gel. (B) Subunit–subunit interactions are stable at 25°C in 1.5% SDS, however the interactions are disrupted by heating the samples to 95°C (8). Periplasmic extracts were run on SDS/PAGE after treatment at 25°C (odd lanes) or 95°C (even lanes). The PapA subunit complexes were visualized by Western blot analysis. Note that with wild-type PapA coexpressed with PapD, subunit complexes migrating with an apparent mobility of dimers, trimers, and tetramers are resolved at 25°C (lane 3). The Tyr-162 substitution to leucine blocks the formation of PapA multimers (lane 11) while the Gly-150 substitution to threonine seems to favor stable trimer formation (lane 17).
Dependence of Subunit Oligomerization on the Beta Zipper.
The effect of the point mutations on the subunit–subunit interactions critical in forming the pilus organelle were investigated by SDS/PAGE and Western blotting of periplasmic extracts after coexpressing PapD with mutant or wild-type PapA in a degP− strain. PapA multimers can be visualized in periplasmic extracts after SDS/PAGE at 25°C, because PapA–PapA but not PapD–PapA associations are stable in SDS at 25°C, whereas both associations are broken in SDS at 95°C (6). All complexes were dissociated at 95°C, both in the absence (Fig. 3B, lanes 2, 6, 10, and 14) and in the presence (Fig. 3B, lanes 4, 8, 12, 16, and 18) of PapD. At 25°C in the presence of PapD, anti-PapA antisera detected oligomeric species of wild-type PapA in the form of dimers, trimers, and tetramers (Fig. 3B, lane 3). In strains not expressing PapD, import of PapA into the periplasm was inefficient and the formation of stable oligomers was greatly reduced for wild-type as well as mutant PapAs (Fig. 3B, lanes 1, 5, 9, and 13).
Mutant PapAs showed differing abilities to oligomerize in the presence of PapD. The Y162F mutant had little or no effect on the formation of PapA oligomers, whereas Y162L abolished the formation of PapA oligomers (Fig. 3B, lane 11). The G150T mutant greatly reduced the presence of AA and AAAA, but maintained a stable AAA (Fig. 3B, lane 17). These results correlate with the ability of G150A and Y162F mutants to form pili in vivo, albeit at reduced levels, whereas G150T and Y162L mutants abolish the formation of pili (data not shown).
Disruption of Packaging Interactions Change the Helical Symmetry of Pili.
The pilus has a rise per subunit of 7.58 Å and 3.279 u/t of the helix (4, 5). The pitch of a helix is defined to be the rise along the helical axis for a complete rotation (360°); the pitch of P pili is, therefore, 24.87 Å (= 7.58 Å rise/subunit 3.279 u/t). If the number of u/t is increased by the G150T PapA mutation, which is unable to form mature pili, addition of a fourth subunit could be sterically prohibited by the first subunit of the adjacent turn. This could explain the occurrence of AAA complexes and the absence of AAAA complexes as seen in Fig. 3B. To investigate this hypothesis and to assess the role of individual amino acids in the subunit–subunit interactions in the quaternary structure of the pilus, changes in the helical symmetry of whole pili, purified from the bacterial cell surface, with point mutations in PapA were determined by Fourier transformation of electron microscopic data by analyzing segments of full-length pili. The helical symmetry of Y162F PapA pili remained essentially unchanged from the wild-type that has 3.279 ± 0.003 u/t and a pitch of 24.87 ± 0.41 Å (n = 21; Fig. 4A); Y162F PapA pili have 3.281 ± 0.006 u/t and a pitch of 24.77 ± 0.87 Å (n = 27; Fig. 4B). Student’s t tests indicated that the differences in symmetry and pitch between wild-type and Y162F PapA pili were not significant: P > 0.5 and P > 0.12, respectively. G150A PapA pili, however, had altered symmetry, as well as increased standard deviations: 3.287 ± 0.009 u/t, with a pitch of 24.67 ± 1.18 Å (n = 32; Fig. 4C). Although the standard deviation of the observed u/t for G150A PapA pili is greater than the difference between the mean values of these pili and wild-type, the number of observations was adequate to distinguish between the means of these two distributions; the use of Student’s t tests indicates that their means are significantly different with a probability >0.999. The observed difference in u/t between wild-type and G150A PapA pili corresponds to a relative movement of interacting subunits of not more than 0.6 Å. The breadths of the observed distributions suggest that the turn-to-turn interaction of the G150A mutant is less rigid than wild type and most likely reflects involvement of G150 in the unique surface on the PapA subunit required for the formation of a right-handed helical cylinder.
Figure 4.
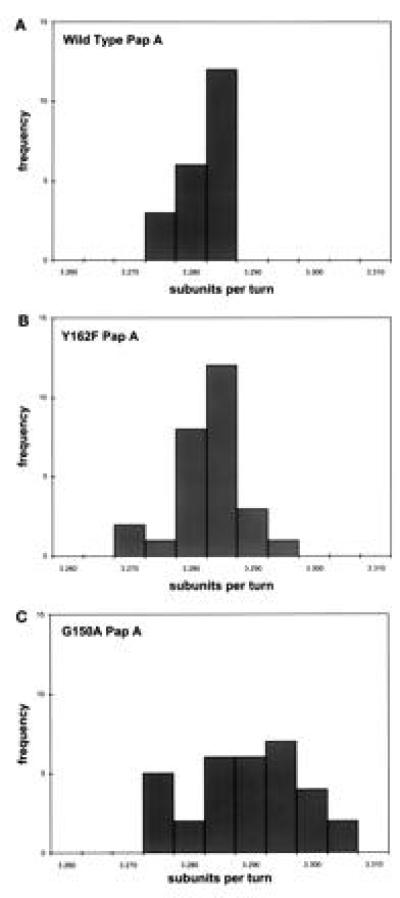
G150A-PapA subunit alters the helical symmetry of the Pap pilus rod. The helical symmetry of pili purified from the bacterial cell surface containing wild-type (A), Y162F (B), and (C) G150A PapA was calculated as described (19). The symmetry of the Y162F pili is unchanged from wild type: 3.281 ± 0.006 with a helical pitch of 24.77 ± 0.87 Å (n = 27) vs. 3.279 ± 0.003 u/t with a helical pitch of 24.87 ± 0.41 Å (n = 21), respectively. The symmetry of the G150A pili is more variable and has a tighter twist, 3.287 ± 0.009 u/t and a helical pitch of 24.67 ± 1.18 Å (n = 32); i.e., this mutant has a larger standard deviation and more u/t of the helix than wild-type pili.
DISCUSSION
Pilus biogenesis is a useful model to investigate one of the most basic problems in molecular biology: the control of the assembly of multimeric structures from monomeric subunits (1, 2). Building a pilus organelle involves six distinct structural subunits that interact via complementary surfaces to form two separate subassemblies that are joined together into composite heteropolymeric fibers. The architecture of each subassembly is determined by the structural characteristics of each subunit type. For example, PapA subunits self-associate into a linear polymer that is coiled into a right-handed helical rod, whereas PapE subunits self-associate into an open helical linear fiber that is joined to the distal ends of pilus rods via the PapK adaptor protein (6). Prior to the assembly of the pilus organelle, the subunits are prevented from associating with each other by the PapD chaperone (11). PapD binds to subunits as (or immediately after) they emerge from the cytoplasmic membrane forming periplasmic chaperone–subunit complexes that are targeted to the outer membrane assembly site, PapC, where subunits polymerize to form mature pili. We reconstituted the rod and tip subassemblies from purified PapD–PapA and PapD–PapE complexes, respectively, demonstrating in vitro that dissociation of the chaperone uncaps interactive surfaces on subunits that drive their assembly into the growing fiber. Using mutant PapA derivatives, we demonstrated that the C-terminal beta zipper motif of the PapA subunit forms part of the complementary surface necessary for the oligomerization and packaging of PapA subunits into helical rods. This finding explains the molecular basis of how the PapD chaperone controls the development of pilus fibers: PapD caps a critical assembly surface at the C-terminal beta zipper of the subunit. This surface is exposed following chaperone uncapping and provides an assembly template for the incorporation of the next subunit.
The discovery that DE and DA chaperone–subunit complexes support the development of tip and rod subassemblies, respectively, suggests that although the PapC usher is required for pilus formation in vivo, it is not required to mediate the subunit–subunit interactions required for the formation of an organellar subassembly. After chaperone–subunit dissociation caused by freeze–thaw in vitro, subunits with self-associating properties are capable of forming organelles. Thus, we propose that after chaperone uncapping, whether mediated by PapC in vivo or freeze–thaw conditions in vitro, assembly may proceed spontaneously. The function of PapC in vivo may be to facilitate the exchange of subunits from a high free energy interaction with the chaperone to a lower free energy subunit–subunit interaction in the quaternary structure of the pilus. Earlier findings in the type 1 pilus system demonstrated that once subunits are polymerized into the type 1 pilus, the surface recognized by the periplasmic chaperone is unavailable, since it is buried in the subunit–subunit interface or has undergone an a conformational change (22).
PapA is the only P pilus subunit that possesses an interactive surface capable of packing into a helical cylinder. Bullitt and Makowski recently demonstrated that the pilus rod is a thread-like PapA fibrillar polymer packaged into a right-handed helical rod (5). Each structural subunit in the pilus makes at least two interactions that are best described as head-to-tail interactions, defining the thread-like PapA and PapE fibrillar polymers. That is, the head of the nth subunit is attached to the tail of the n + 1st, and the tail of the nth subunit is attached to the head of the n − 1st subunit. PapA subunits, however, make additional packaging interactions that transform the thin fibrillar conformation of the PapA polymer into the right-handed helical rod, connecting the nth subunit with the n ± 3rd and/or n ± 4th subunits that are one helical turn away (E.B. and L.M., unpublished work). The existence of a ternary DA2 complex and higher order complexes argues that PapD does not cap all the interactive surfaces on this subunit. Both the Y162L and G150T mutations abolish subunit–subunit interactions critical to the development of pilus organelles, but only the G150T mutation reduced the A-A interaction in the DA2 complex. The A–A interaction in the DA2 complex most likely reflects, in part, the unique surface present only on the PapA subunit that allows packaging into a right-handed helical cylinder. The symmetry results are consistent with our hypothesis that PapA has a unique surface involved in formation of a closed helical structure. Pilus rods comprised of G150A PapA have an altered helical symmetry with azimuthal disorder, as indicated by the significant change in u/t as compared with wild-type, whereas the pitch remained minimally altered, if at all (P = 0.046). Mutations in G150 also reduced or abolished the A–A interaction in the DA2 complex indicating that the A–A interaction in the DA2 complex most likely reflects, in part, the unique surface present only on the PapA subunit that allows packaging into a right-handed helical cylinder. G150 is situated such that it does not make any interactions with PapD (15), and accordingly the G150A and G150T mutations did not affect the formation of the DA complex (Fig. 3A). In contrast, Y162 is a critical part of the beta zipper motif on subunits, and the Y162F and Y162L mutations consequently reduce and abolish, respectively, DA complex formation. However, mutations in Y162 had no effect on the A–A interaction in the DA2 complex, suggesting that Y162 is not critical for the so-called packaging interactions (Fig. 3A). Consistent with this hypothesis was the finding that pilus rods comprised of Y162F PapA had a helical symmetry indistinguishable from wild-type rods.
Consistent with a role in the packaging of the PapA rod into a helical cylinder is the ability of G150T PapA to form SDS resistant AAA complexes but not AAAA complexes. The fourth subunit added to a pilus rod would represent the start of the second turn of the rod since there are 3.279 u/t of the helix (4, 5). We suggests that the incorporation of a fourth subunit into the pilus is precluded due to structural alterations in the subunit caused by the G150T substitution. Consequently, the mutation abolishes the formation of pilus rods.
In conclusion, our data argue that the PapD chaperone modulates the assembly of the pilus by capping the C-terminal motif of pilus subunits and subsequently delivering and uncapping the subunit at the usher assembly site (see model, Fig. 1). This capping function prevents premature interaction of subunits in the periplasm and allows for controlled interaction of subunits at the outer membrane assembly site. Uncapping of the chaperone-protected subunit C terminus was mimicked in vitro by freeze–thaw techniques; furthermore, the fate of the uncapped subunits in organelle development in the in vitro assembly system was the same as their fate in vivo. The ability to reconstitute pilus assembly in vitro will allow further dissection of pilus biogenesis and aid in the understanding of how the fibrillum structure is adapted to the rod via the pilus adapter proteins. Future studies should aim at elucidating the molecular mechanism of how the usher facilitates the chaperone uncapping process.
Acknowledgments
We thank Mary-Jane Lombardo, K. Dodson, and S. Knight for helpful advice in writing this manuscript. This work was supported by National Institutes of Health Grant R01A129549 and MedImmune, Inc. (S.J.H.) and by National Institutes of Health Fellowship F32AI08665 (C.H.J.).
Footnotes
Abbreviation: u/t, subunits per turn.
References
- 1.Hultgren S J, Abraham S N, Caparon M G, Falk P, St. Geme J W, III, Normark S. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- 2.Hultgren S J, Normark S, Abraham S N. Annu Rev Microbiol. 1991;45:383–415. doi: 10.1146/annurev.mi.45.100191.002123. [DOI] [PubMed] [Google Scholar]
- 3.Kuehn M J, Heuser J, Normark S, Hultgren S J. Nature (London) 1992;356:252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- 4.Gong M, Makowski L. J Mol Biol. 1992;228:735–742. doi: 10.1016/0022-2836(92)90860-m. [DOI] [PubMed] [Google Scholar]
- 5.Bullitt E, Makowski L. Nature (London) 1995;373:164–167. doi: 10.1038/373164a0. [DOI] [PubMed] [Google Scholar]
- 6.Jacob-Dubuisson F, Heuser J, Dodson K, Normark S, Hultgren S J. EMBO J. 1993;12:837–847. doi: 10.1002/j.1460-2075.1993.tb05724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts J A, Marklund B-I, Ilver D, Haslam D, Kaack M B, Baskin G, Louis M, Mollby R, Winberg J, Normark S. Proc Natl Acad Sci USA. 1994;91:11889–11893. doi: 10.1073/pnas.91.25.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Striker R, Jacob-Dubuisson F, Frieden C, Hultgren S J. J Biol Chem. 1994;269:12233–12239. [PubMed] [Google Scholar]
- 9.Holmgren A, Brändén C. Nature (London) 1989;342:248–251. doi: 10.1038/342248a0. [DOI] [PubMed] [Google Scholar]
- 10.Hultgren S J, Lindberg F, Magnusson G, Kihlberg J, Tennent J M, Normark S. Proc Natl Acad Sci USA. 1989;86:4357–4361. doi: 10.1073/pnas.86.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuehn M J, Normark S, Hultgren S J. Proc Natl Acad Sci USA. 1991;88:10586–10590. doi: 10.1073/pnas.88.23.10586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dodson K W, Jacob-Dubuisson F, Striker R T, Hultgren S J. Proc Natl Acad Sci USA. 1993;90:3670–3674. doi: 10.1073/pnas.90.8.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmgren A, Kuehn M J, Brändén C-I, Hultgren S J. EMBO J. 1992;11:1617–1622. doi: 10.1002/j.1460-2075.1992.tb05207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacob-Dubuisson F, Pinkner J, Xu Z, Striker R, Padmanhaban A, Hultgren S J. Proc Natl Acad Sci USA. 1994;91:11552–11556. doi: 10.1073/pnas.91.24.11552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuehn M J, Ogg D J, Kihlberg J, Slonim L N, Flemmer K, Bergfors T, Hultgren S J. Science. 1993;262:1234–1241. doi: 10.1126/science.7901913. [DOI] [PubMed] [Google Scholar]
- 16.Slonim L N, Pinkner J S, Brändén C I, Hultgren S J. EMBO J. 1992;11:4747–4756. doi: 10.1002/j.1460-2075.1992.tb05580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strauch K L, Johnson K, Beckwith J. J Bacteriol. 1989;171:2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison H G, Desrosiers R C. BioTechniques. 1993;14:454–457. [PubMed] [Google Scholar]
- 19.Klug A, Crick G H C, Wycoff H W. Acta Crystallogr. 1958;11:199–213. [Google Scholar]
- 20.Maiti S N, DesGroselillers L, Fairbrother J M, Harel J. Mol Pathol. 1994;16:15–25. doi: 10.1006/mpat.1994.1002. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Jones C H, Haslam D, Dodson K, Kihlberg J, Hultgren S J. Mol Microbiol. 1995;16:1011–1020. doi: 10.1111/j.1365-2958.1995.tb02326.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones C H, Pinkner J S, Roth R, Heuser J, Nicholoes A V, Abraham S N, Hultgren S J. Proc Natl Acad Sci USA. 1995;92:2081–2085. doi: 10.1073/pnas.92.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]


