Abstract
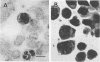
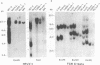
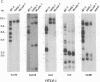
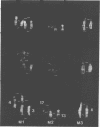
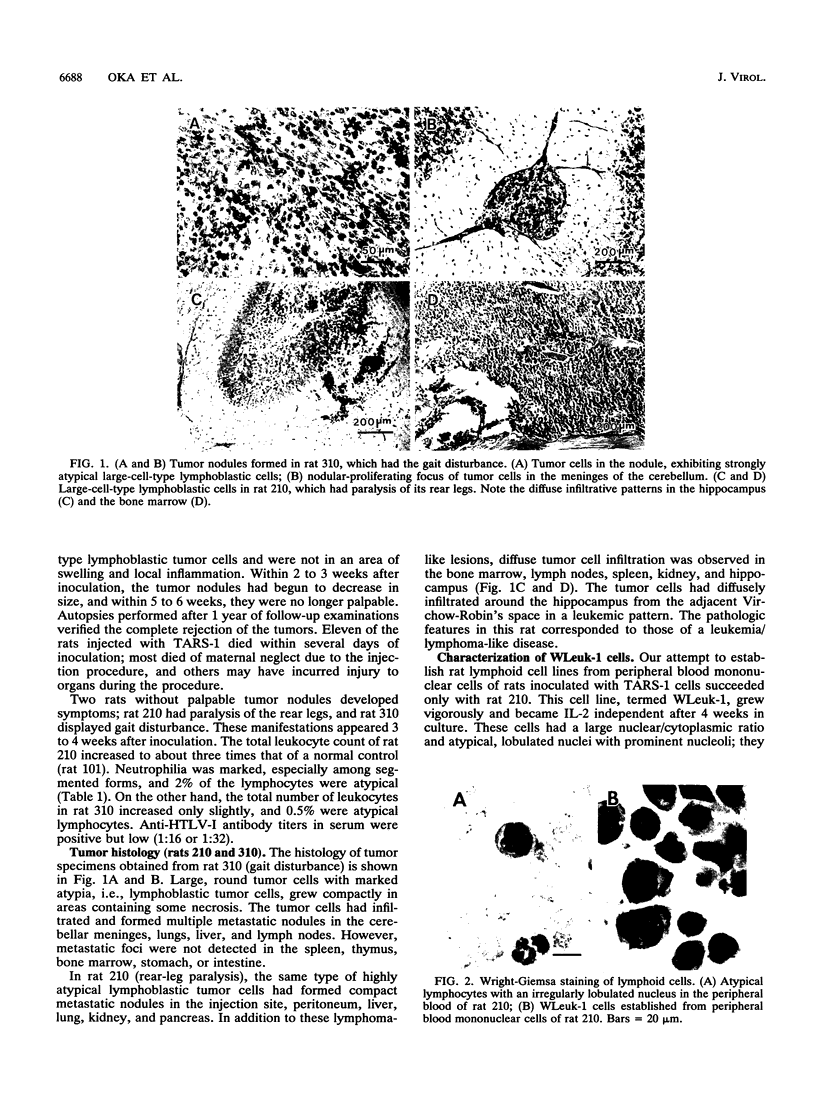
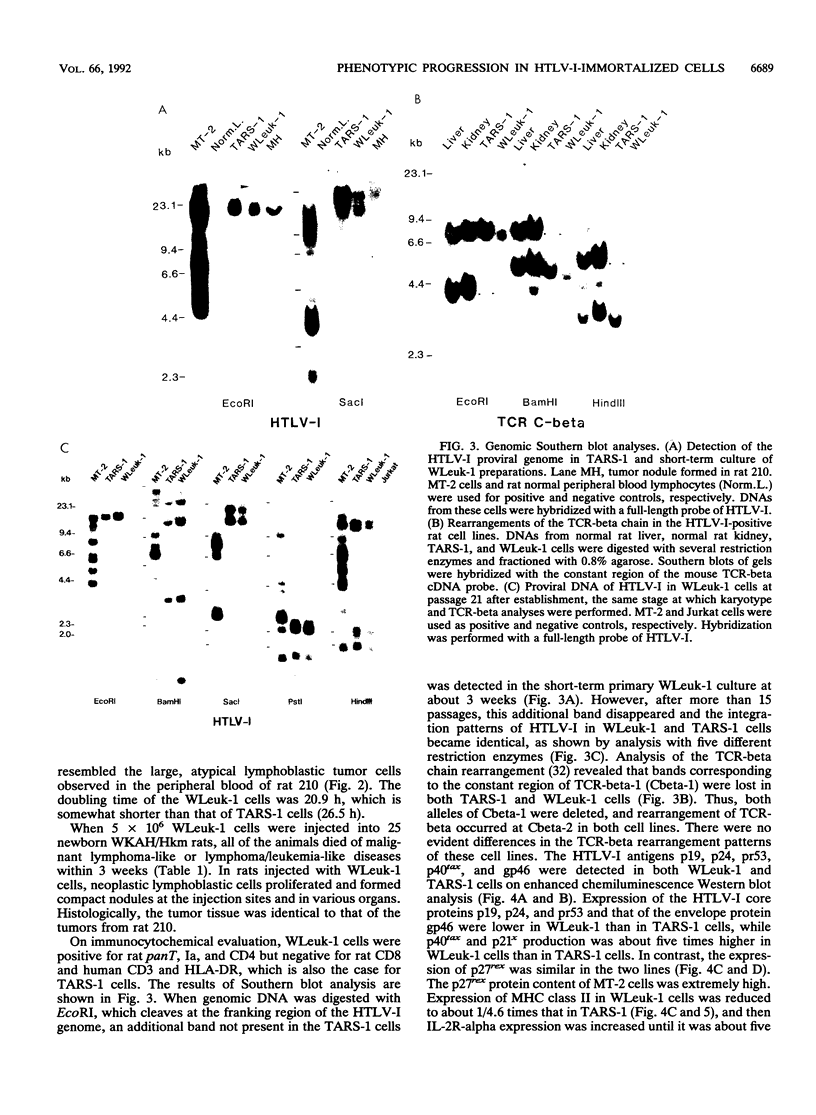
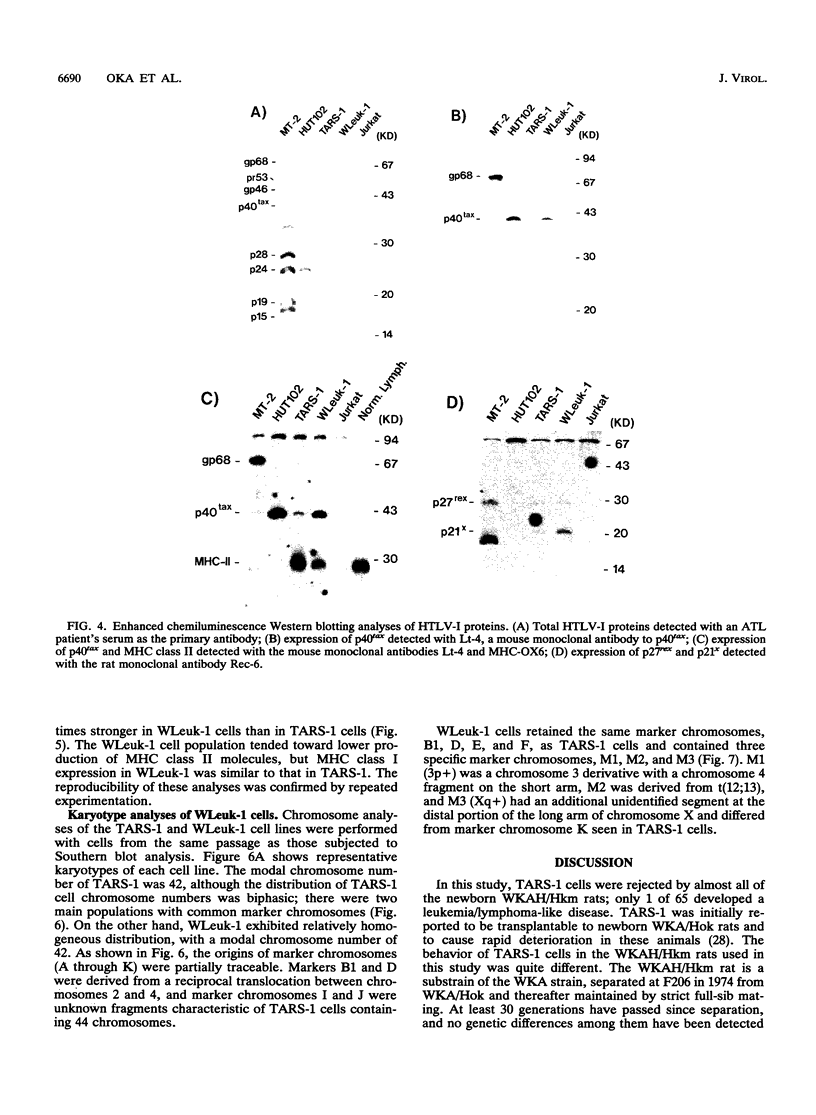
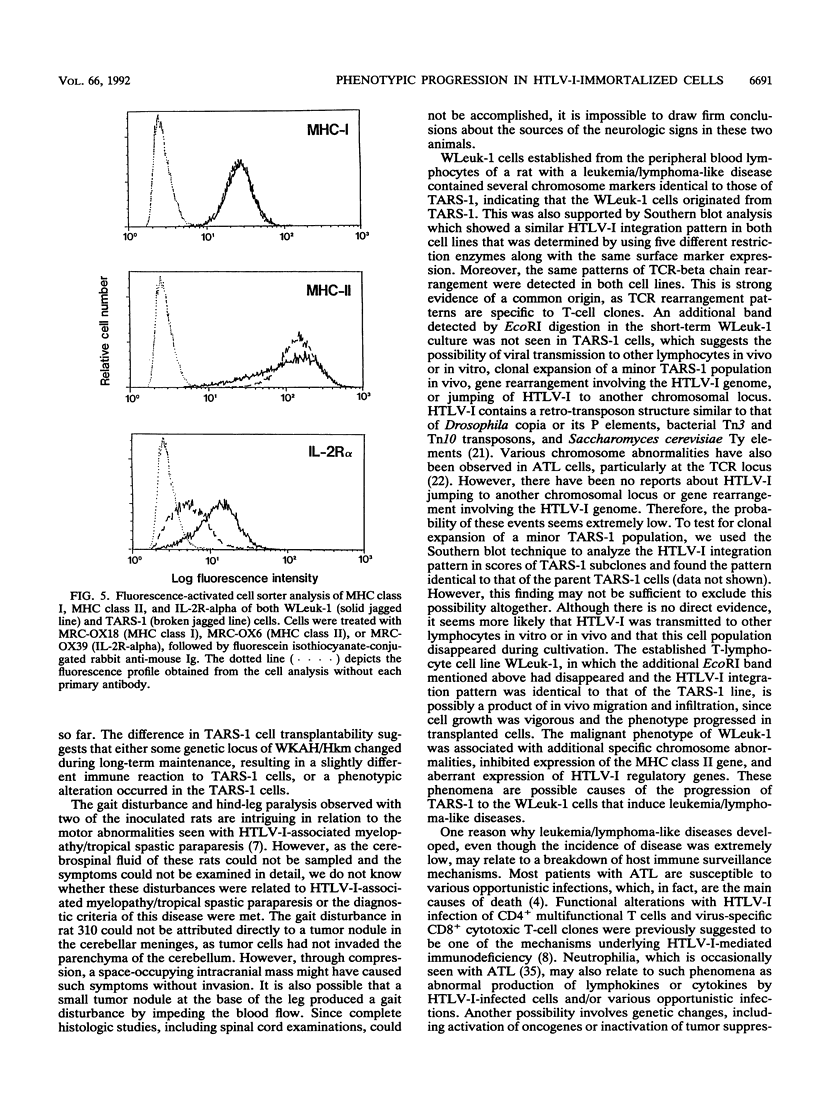
Rat lymphoid cells, TARS-1, immortalized by coculture with adult T-cell leukemia cells, were intraperitoneally injected into 65 newborn, inbred WKAH/Hkm rats. In most of the rats, tumor nodules were discernible 7 to 15 days after transplantation but were completely rejected within 5 to 6 weeks. Two rats with no tumor nodules exhibited gait disturbances and paralysis of the hind legs 3 to 4 weeks after transplantation. Histological and hematological examinations revealed that a lymphoma/leukemia-like disease had developed in one of the two rats, and the T-lymphoid cell line WLeuk-1 was established from peripheral blood mononuclear cells from this rat. When the WLeuk-1 cells were transplanted into newborn WKAH/Hkm rats, the animals died of a lymphoma/leukemia-like disease within several weeks after transplantation, in contrast to their rejection of the TARS-1 cells. Southern blot and karyotype analyses revealed that WLeuk-1 cells had retained the marker chromosomes and human T-lymphotropic virus type I (HTLV-I) integration patterns of the parent cell line, TARS-1. The additional specific chromosome abnormalities 3p+,t (12;13), and Xq+ were found in the WLeuk-1 cells. Moreover, the expression of HTLV-I structural proteins was slightly depressed in WLeuk-1 cells, while that of the transacting factors p40tax and p21x, but not that of p27rex, was enhanced about fivefold compared with that in TARS-1. The transactivating function of p40tax was intact in WLeuk-1, as evidenced by enhanced interleukin-2 receptor alpha chain expression. These results suggest that aberrant expression of HTLV-I regulatory genes and alteration of cellular genes were associated with the phenotypic progression of the WLeuk-1 cell line.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagi T., Takata H., Ohtsuki Y., Takahashi K., Oka T., Yano S., Miyoshi I. Transformation of hamster spleen lymphocytes by human T-cell leukemia virus type I. Int J Cancer. 1986 May 15;37(5):775–779. doi: 10.1002/ijc.2910370520. [DOI] [PubMed] [Google Scholar]
- Akagi T., Takeda I., Oka T., Ohtsuki Y., Yano S., Miyoshi I. Experimental infection of rabbits with human T-cell leukemia virus type I. Jpn J Cancer Res. 1985 Feb;76(2):86–94. [PubMed] [Google Scholar]
- Bunn P. A., Jr, Schechter G. P., Jaffe E., Blayney D., Young R. C., Matthews M. J., Blattner W., Broder S., Robert-Guroff M., Gallo R. C. Clinical course of retrovirus-associated adult T-cell lymphoma in the United States. N Engl J Med. 1983 Aug 4;309(5):257–264. doi: 10.1056/NEJM198308043090501. [DOI] [PubMed] [Google Scholar]
- Eguchi T., Kubonishi I., Daibata M., Yano S., Imamura J., Ohtsuki Y., Miyoshi I. Serial transplantation of an HTLV-I-transformed hamster lymphoid cell line into hamsters. Int J Cancer. 1988 Jun 15;41(6):868–872. doi: 10.1002/ijc.2910410617. [DOI] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Inatsuki A., Yasukawa M., Kobayashi Y. The effect of human T cell leukaemia virus type I infection on a herpes simplex virus-specific CD8+ cytotoxic T cell clone. Br J Haematol. 1991 Mar;77(3):311–314. doi: 10.1111/j.1365-2141.1991.tb08576.x. [DOI] [PubMed] [Google Scholar]
- Inoue J., Seiki M., Taniguchi T., Tsuru S., Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986 Nov;5(11):2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Yoshida M., Seiki M. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3653–3657. doi: 10.1073/pnas.84.11.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeang K. T., Widen S. G., Semmes O. J., 4th, Wilson S. H. HTLV-I trans-activator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science. 1990 Mar 2;247(4946):1082–1084. doi: 10.1126/science.2309119. [DOI] [PubMed] [Google Scholar]
- Kataoka R., Takehara N., Iwahara Y., Sawada T., Ohtsuki Y., Dawei Y., Hoshino H., Miyoshi I. Transmission of HTLV-I by blood transfusion and its prevention by passive immunization in rabbits. Blood. 1990 Oct 15;76(8):1657–1661. [PubMed] [Google Scholar]
- Kawano F., Yamaguchi K., Nishimura H., Tsuda H., Takatsuki K. Variation in the clinical courses of adult T-cell leukemia. Cancer. 1985 Feb 15;55(4):851–856. doi: 10.1002/1097-0142(19850215)55:4<851::aid-cncr2820550424>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Levan G. Nomenclature for G-bands in rat chromosomes. Hereditas. 1974;77(1):37–52. doi: 10.1111/j.1601-5223.1974.tb01352.x. [DOI] [PubMed] [Google Scholar]
- Miyatake S., Seiki M., Malefijt R. D., Heike T., Fujisawa J., Takebe Y., Nishida J., Shlomai J., Yokota T., Yoshida M. Activation of T cell-derived lymphokine genes in T cells and fibroblasts: effects of human T cell leukemia virus type I p40x protein and bovine papilloma virus encoded E2 protein. Nucleic Acids Res. 1988 Jul 25;16(14A):6547–6566. doi: 10.1093/nar/16.14.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Yoshimoto S., Akagi T., Ohtsuki Y., Shiraishi Y., Nagata K., Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981 Dec 24;294(5843):770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- Nagata K., Ohtani K., Nakamura M., Sugamura K. Activation of endogenous c-fos proto-oncogene expression by human T-cell leukemia virus type I-encoded p40tax protein in the human T-cell line, Jurkat. J Virol. 1989 Aug;63(8):3220–3226. doi: 10.1128/jvi.63.8.3220-3226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T., Ohtsuki Y., Sonobe H., Furihata M., Miyoshi I. Suppressive effects of interferons on the production and release of human T-lymphotropic virus type-I (HTLV-I). Arch Virol. 1990;115(1-2):63–73. doi: 10.1007/BF01310623. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Ohno Y., Tsugane S., Watanabe S., Shimoyama M., Tajima K., Miwa M., Shimotohno K. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989 Mar;80(3):191–195. doi: 10.1111/j.1349-7006.1989.tb02289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D. C. Molecular mechanisms regulating Drosophila P element transposition. Annu Rev Genet. 1990;24:543–578. doi: 10.1146/annurev.ge.24.120190.002551. [DOI] [PubMed] [Google Scholar]
- Sadamori N., Kusano M., Nishino K., Tagawa M., Yao E., Yamada Y., Amagasaki T., Kinoshita K., Ichimaru M. Abnormalities of chromosome 14 at band 14q11 in Japanese patients with adult T-cell leukemia. Cancer Genet Cytogenet. 1985 Jul;17(3):279–282. doi: 10.1016/0165-4608(85)90019-6. [DOI] [PubMed] [Google Scholar]
- Satoh H., Yoshida M. C., Sasaki M. High resolution chromosome banding in the Norway rat, Rattus norvegicus. Cytogenet Cell Genet. 1989;50(2-3):151–154. doi: 10.1159/000132747. [DOI] [PubMed] [Google Scholar]
- Seto A., Kawanishi M., Matsuda S., Ogawa K., Miyoshi I. Adult T cell leukemia-like disease experimentally induced in rabbits. Jpn J Cancer Res. 1988 Mar;79(3):335–341. doi: 10.1111/j.1349-7006.1988.tb01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi R., Mihara K., Maeda S., Yamaguchi T., Chen H. L., Aoyama N., Murao S., Hatanaka M., Sugiyama T. Secondary activation of c-abl may be related to translocation to the nucleolar organizer region in an in vitro cultured rat leukemia cell line (K3D). Proc Natl Acad Sci U S A. 1986 Feb;83(4):1079–1083. doi: 10.1073/pnas.83.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Yoshida A., Takayama Y., Tsujimoto H., Tsujimoto A., Hayami M., Tozawa H. Heterogeneity of antigen molecules recognized by anti-tax1 monoclonal antibody Lt-4 in cell lines bearing human T cell leukemia virus type I and related retroviruses. Jpn J Cancer Res. 1990 Mar;81(3):225–231. doi: 10.1111/j.1349-7006.1990.tb02554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateno M., Kondo N., Itoh T., Chubachi T., Togashi T., Yoshiki T. Rat lymphoid cell lines with human T cell leukemia virus production. I. Biological and serological characterization. J Exp Med. 1984 Apr 1;159(4):1105–1116. doi: 10.1084/jem.159.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschachler E., Robert-Guroff M., Gallo R. C., Reitz M. S., Jr Human T-lymphotropic virus I-infected T cells constitutively express lymphotoxin in vitro. Blood. 1989 Jan;73(1):194–201. [PubMed] [Google Scholar]
- Tsujimoto H., Seiki M., Nakamura H., Watanabe T., Sakakibara I., Sasagawa A., Honjo S., Hayami M., Yoshida M. Adult T-cell leukemia-like disease in monkey naturally infected with simian retrovirus related to human T-cell leukemia virus type I. Jpn J Cancer Res. 1985 Oct;76(10):911–914. [PubMed] [Google Scholar]
- Williams C. B., Blankenhorn E. P., Byrd K. E., Levinson G., Gutman G. A. Organization and nucleotide sequence of the rat T cell receptor beta-chain complex. J Immunol. 1991 Jun 15;146(12):4406–4413. [PubMed] [Google Scholar]
- Wong-Staal F., Gallo R. C. Human T-lymphotropic retroviruses. Nature. 1985 Oct 3;317(6036):395–403. doi: 10.1038/317395a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Hayami M., Komuro A., Schneider J., Hunsmann G., Okada M., Hinuma Y. Experimental infection of cynomolgus monkeys with a human retrovirus, adult T-cell leukemia virus. Med Microbiol Immunol. 1984;173(1):57–64. doi: 10.1007/BF02123570. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Hattori T., Asou N., Nishimura H., Kawano F., Yodoi J., Takatsuki K. Absolute neutrophilia in adult T cell leukemia. Jpn J Cancer Res. 1986 Sep;77(9):858–861. [PubMed] [Google Scholar]
- Yodoi J., Okada M., Tagaya Y., Teshigawara K., Fukui K., Ishida N., Ikuta K., Maeda M., Honjo T., Osawa H. Rat lymphoid cell lines producing human T cell leukemia virus. II. Constitutive expression of rat interleukin 2 receptor. J Exp Med. 1985 May 1;161(5):924–934. doi: 10.1084/jem.161.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiki T., Kondo N., Chubachi T., Tateno M., Togashi T., Itoh T. Rat lymphoid cell lines with HTLV-I production. III. Transmission of HTLV-I into rats and analysis of cell surface antigens associated with HTLV-I. Arch Virol. 1987;97(3-4):181–196. doi: 10.1007/BF01314420. [DOI] [PubMed] [Google Scholar]