Abstract
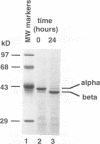
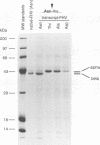
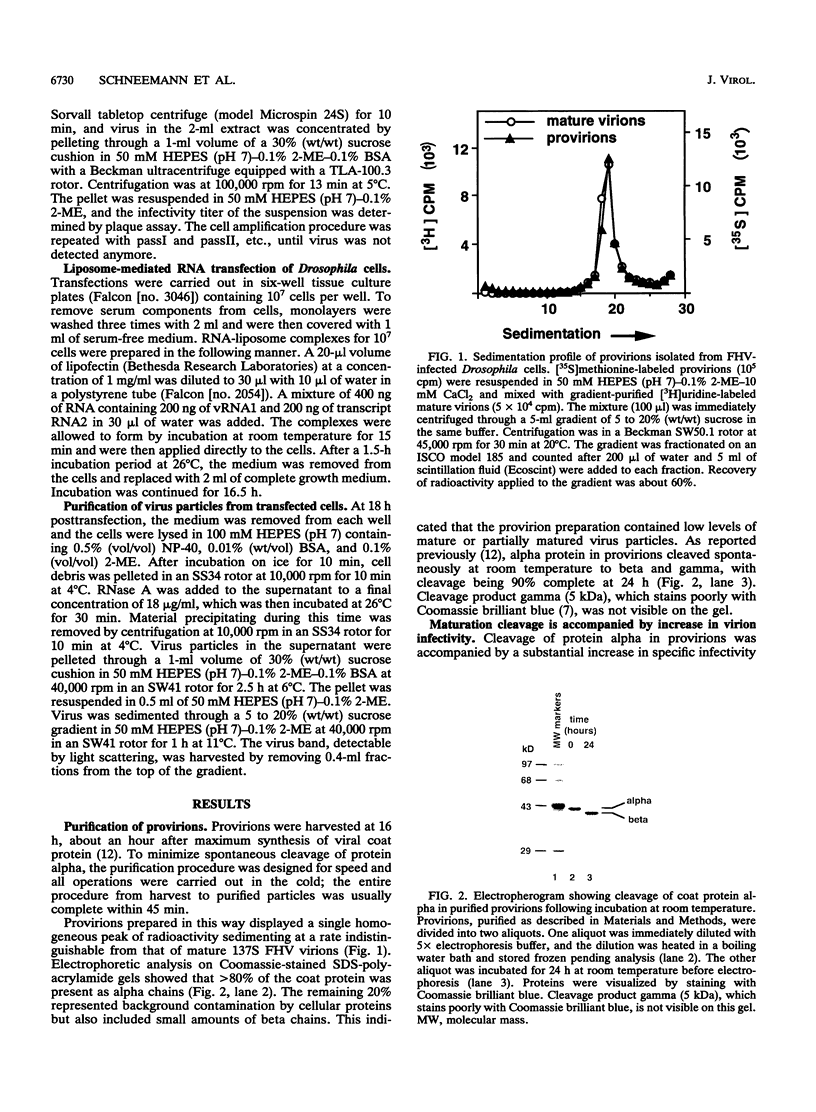
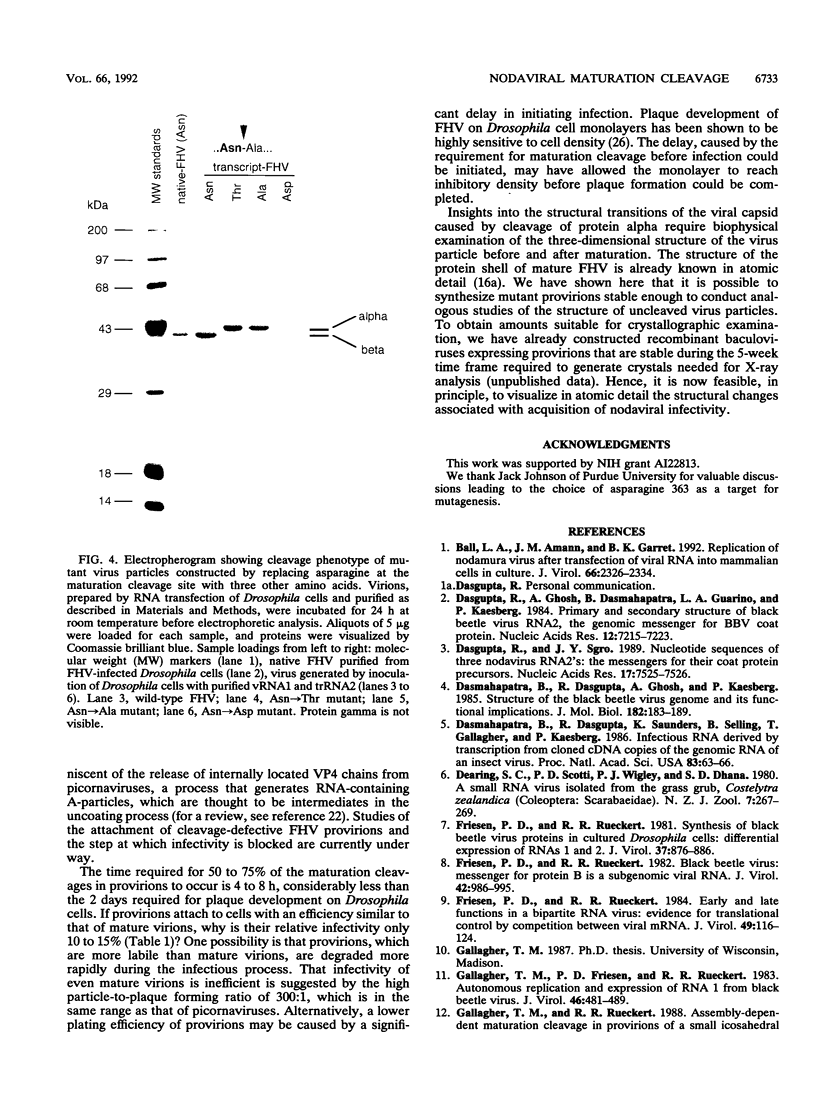
Nodaviral morphogenesis involves formation of labile precursor particles, called provirions, which mature by autocatalytic cleavage of the 407-residue coat precursor protein between asparagine residue 363 and alanine residue 364. It has previously been demonstrated that maturation results in increased physicochemical stability of the virion. We show here that cleavage of coat protein in purified provirions of Flock House virus was accompanied by a five- to eightfold increase in specific infectivity. Cleavage-negative provirions, produced by site-directed mutagenesis of asparagine residue 363 to aspartate, threonine, or alanine, displayed no infectivity above revertant frequencies as measured by plaque assay. All viable revertants (nine of nine) restored asparagine to the mutated position, suggesting high specificity for asparagine at the cleavage site.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball L. A., Amann J. M., Garrett B. K. Replication of nodamura virus after transfection of viral RNA into mammalian cells in culture. J Virol. 1992 Apr;66(4):2326–2334. doi: 10.1128/jvi.66.4.2326-2334.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta R., Ghosh A., Dasmahapatra B., Guarino L. A., Kaesberg P. Primary and secondary structure of black beetle virus RNA2, the genomic messenger for BBV coat protein precursor. Nucleic Acids Res. 1984 Sep 25;12(18):7215–7223. doi: 10.1093/nar/12.18.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta R., Sgro J. Y. Nucleotide sequences of three Nodavirus RNA2's: the messengers for their coat protein precursors. Nucleic Acids Res. 1989 Sep 25;17(18):7525–7526. doi: 10.1093/nar/17.18.7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasmahapatra B., Dasgupta R., Ghosh A., Kaesberg P. Structure of the black beetle virus genome and its functional implications. J Mol Biol. 1985 Mar 20;182(2):183–189. doi: 10.1016/0022-2836(85)90337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasmahapatra B., Dasgupta R., Saunders K., Selling B., Gallagher T., Kaesberg P. Infectious RNA derived by transcription from cloned cDNA copies of the genomic RNA of an insect virus. Proc Natl Acad Sci U S A. 1986 Jan;83(1):63–66. doi: 10.1073/pnas.83.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P. D., Rueckert R. R. Black beetle virus: messenger for protein B is a subgenomic viral RNA. J Virol. 1982 Jun;42(3):986–995. doi: 10.1128/jvi.42.3.986-995.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P. D., Rueckert R. R. Early and late functions in a bipartite RNA virus: evidence for translational control by competition between viral mRNAs. J Virol. 1984 Jan;49(1):116–124. doi: 10.1128/jvi.49.1.116-124.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen P. D., Rueckert R. R. Synthesis of Black Beetle Virus Proteins in Cultured Drosophila Cells: Differential Expression of RNAs 1 and 2. J Virol. 1981 Mar;37(3):876–886. doi: 10.1128/jvi.37.3.876-886.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. M., Friesen P. D., Rueckert R. R. Autonomous replication and expression of RNA 1 from black beetle virus. J Virol. 1983 May;46(2):481–489. doi: 10.1128/jvi.46.2.481-489.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino L. A., Ghosh A., Dasmahapatra B., Dasgupta R., Kaesberg P. Sequence of the black beetle virus subgenomic RNA and its location in the viral genome. Virology. 1984 Nov;139(1):199–203. doi: 10.1016/0042-6822(84)90342-8. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Kaesberg P. Isolation and Characterization of an RNA-Dependent RNA Polymerase from Black Beetle Virus-Infected Drosophila melanogaster Cells. J Virol. 1981 Nov;40(2):379–386. doi: 10.1128/jvi.40.2.379-386.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosur M. V., Schmidt T., Tucker R. C., Johnson J. E., Gallagher T. M., Selling B. H., Rueckert R. R. Structure of an insect virus at 3.0 A resolution. Proteins. 1987;2(3):167–176. doi: 10.1002/prot.340020302. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Newman J. F., Brown F. Further physicochemical characterization of Nodamura virus. Evidence that the divided genome occurs in a single component. J Gen Virol. 1978 Jan;38(1):83–95. doi: 10.1099/0022-1317-38-1-83. [DOI] [PubMed] [Google Scholar]
- Reinganum C., Bashiruddin J. B., Cross G. F. Boolarra virus: a member of the Nodaviridae isolated from Oncopera intricoides (Lepidoptera: Hepialidae). Intervirology. 1985;24(1):10–17. doi: 10.1159/000149613. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K., Kaesberg P. Template-dependent RNA polymerase from black beetle virus-infected Drosophila melanogaster cells. Virology. 1985 Dec;147(2):373–381. doi: 10.1016/0042-6822(85)90139-4. [DOI] [PubMed] [Google Scholar]
- Scherer W. F., Hurlbut H. S. Nodamura virus from Japan: a new and unusual arbovirus resistant to diethyl ether and chloroform. Am J Epidemiol. 1967 Sep;86(2):271–285. doi: 10.1093/oxfordjournals.aje.a120737. [DOI] [PubMed] [Google Scholar]
- Selling B. H., Rueckert R. R. Plaque assay for black beetle virus. J Virol. 1984 Jul;51(1):251–253. doi: 10.1128/jvi.51.1.251-253.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]