Abstract
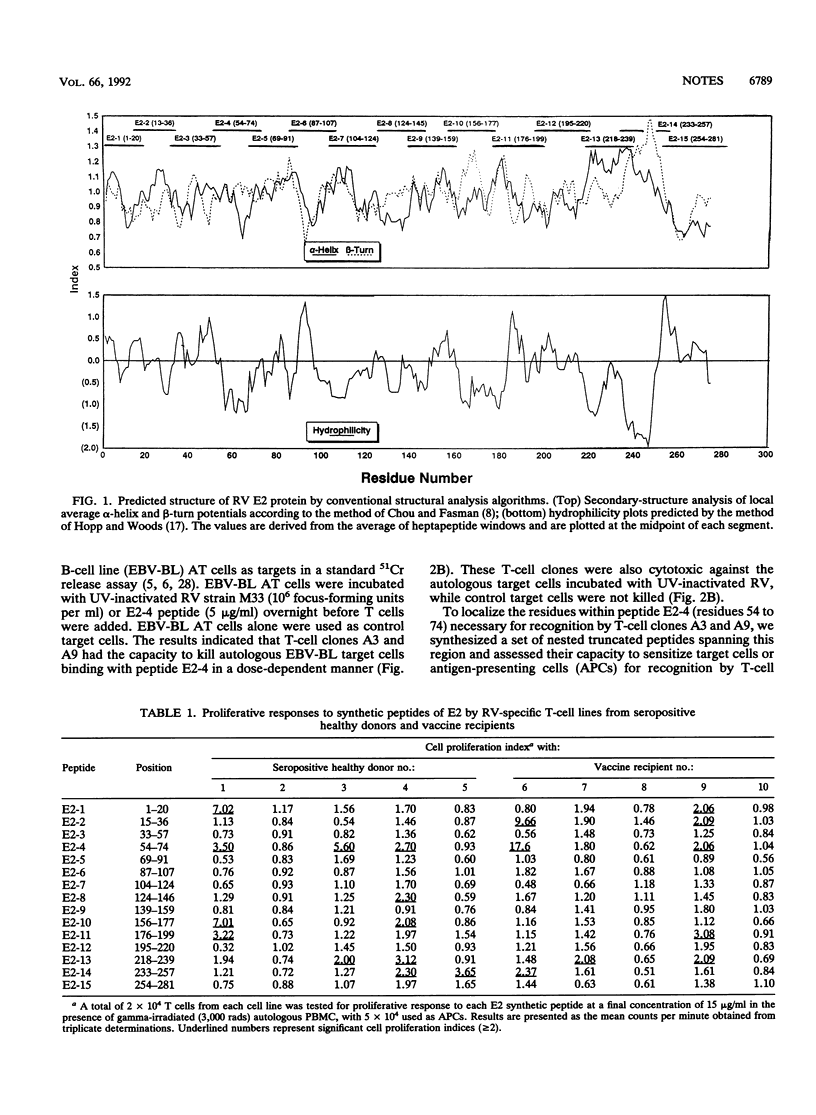
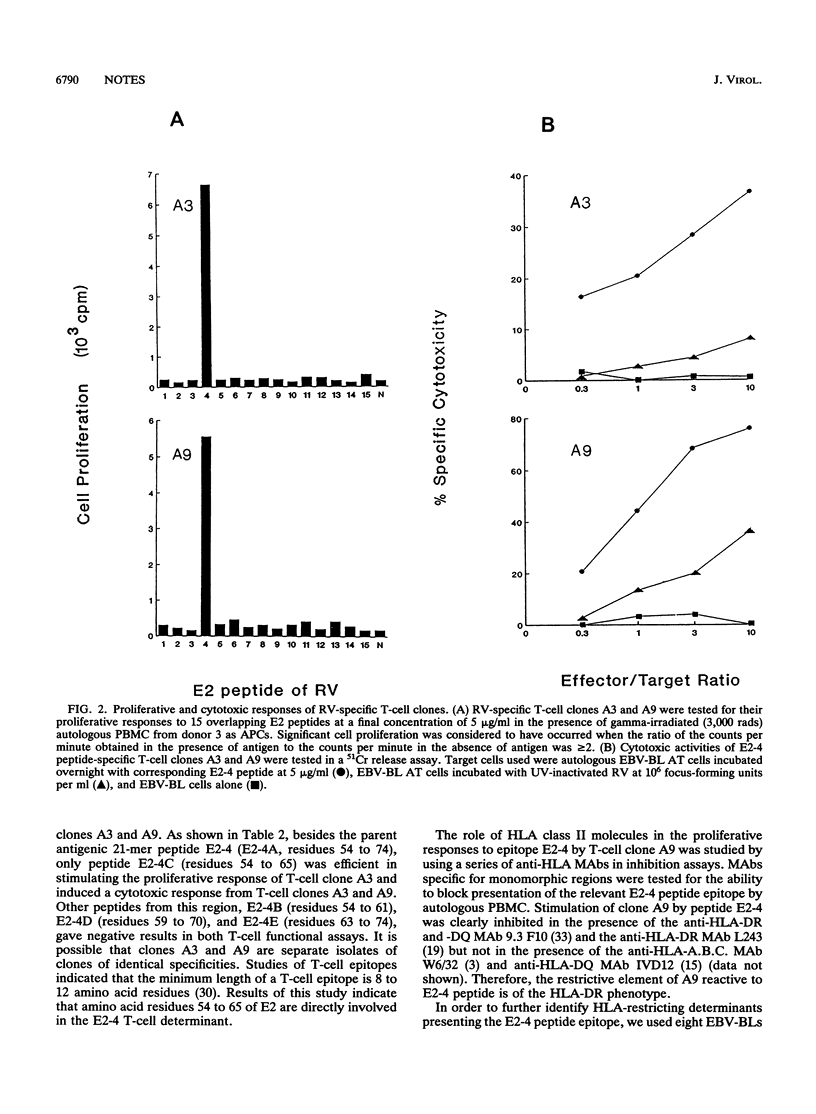
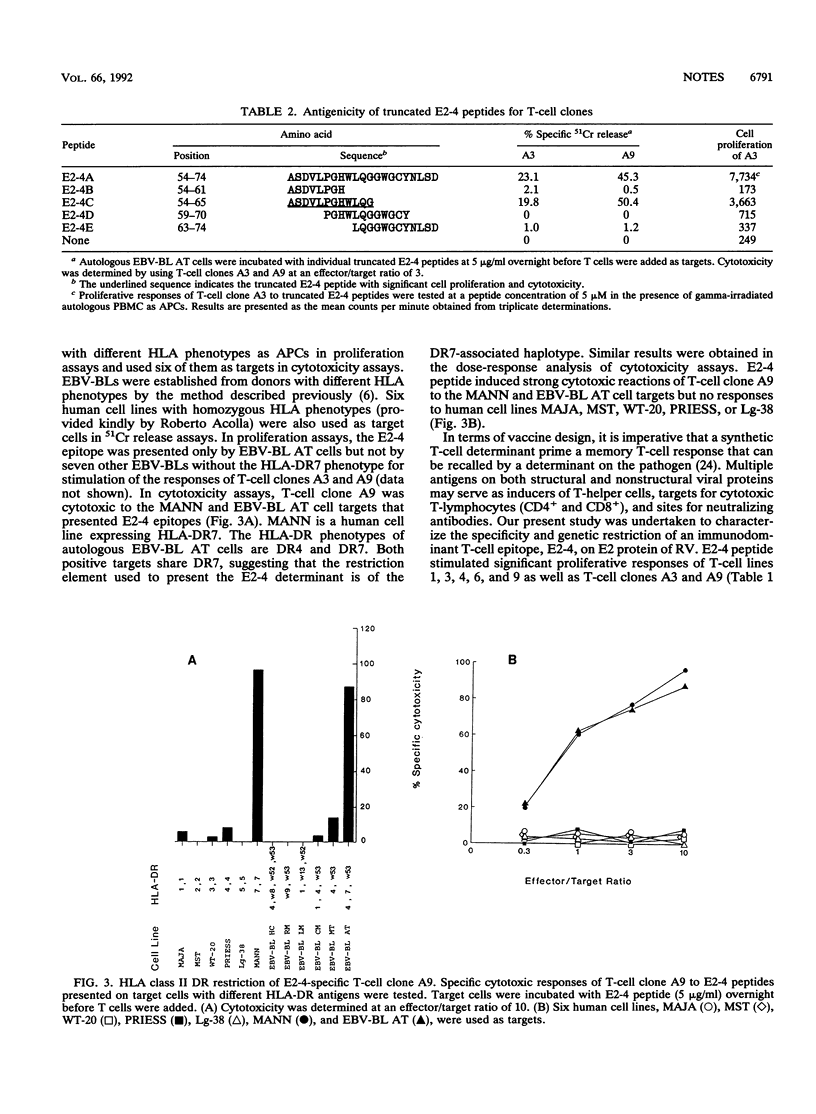
T-cell epitopes on the E2 protein of rubella virus were studied by using 15 overlapping synthetic peptides covering the E2 protein sequence. The most frequently recognized epitopes on E2 were E2-4 (residues 54 to 74), with 5 of 10 tested T-cell lines responding to it. Two CD4+ cytotoxic T-cell cloned isolated from one T-cell line responded strongly in proliferation assays with peptide E2-4 and were cytotoxic to target cells presenting the E2-4 determinant. Truncated peptides contained within the E2-4 peptide sequence were used to define the T-cell determinants. Results indicated that amino acid residues 54 to 65 were directly involved. Human cell lines with different HLA phenotypes were tested for the capacity to present the antigenic determinants. The results suggested that recognition of peptide E2-4 by T-cell clones was associated with HLA DR7.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assaad F., Ljungars-Esteves K. Rubella--world impact. Rev Infect Dis. 1985 Mar-Apr;7 (Suppl 1):S29–S36. doi: 10.1093/clinids/7.supplement_1.s29. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Bart S. W., Stetler H. C., Preblud S. R., Williams N. M., Orenstein W. A., Bart K. J., Hinman A. R., Herrmann K. L. Fetal risk associated with rubella vaccine: an update. Rev Infect Dis. 1985 Mar-Apr;7 (Suppl 1):S95–102. doi: 10.1093/clinids/7.supplement_1.s95. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P. Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol. 1982 Jan;128(1):129–135. [PubMed] [Google Scholar]
- Brown L. R., Nygard N. R., Graham M. B., Bono C., Braciale V. L., Gorka J., Schwartz B. D., Braciale T. J. Recognition of the influenza hemagglutinin by class II MHC-restricted T lymphocytes and antibodies. I. Site definition and implications for antigen presentation and T lymphocyte recognition. J Immunol. 1991 Oct 15;147(8):2677–2684. [PubMed] [Google Scholar]
- Celis E., Ou D., Dietzschold B., Koprowski H. Recognition of rabies and rabies-related viruses by T cells derived from human vaccine recipients. J Virol. 1988 Sep;62(9):3128–3134. doi: 10.1128/jvi.62.9.3128-3134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis E., Ou D., Otvos L., Jr Recognition of hepatitis B surface antigen by human T lymphocytes. Proliferative and cytotoxic responses to a major antigenic determinant defined by synthetic peptides. J Immunol. 1988 Mar 15;140(6):1808–1815. [PubMed] [Google Scholar]
- Chantler J. K., Ford D. K., Tingle A. J. Persistent rubella infection and rubella-associated arthritis. Lancet. 1982 Jun 12;1(8285):1323–1325. doi: 10.1016/s0140-6736(82)92398-4. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., Hui I., Chong P., Gillam S. Nucleotide sequence and in vitro expression of rubella virus 24S subgenomic messenger RNA encoding the structural proteins E1, E2 and C. Nucleic Acids Res. 1987 Apr 10;15(7):3041–3057. doi: 10.1093/nar/15.7.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett P. H., Miller D. C., Green K. Y., Byrd F. I. Structure and function of the rubella virus proteins. Rev Infect Dis. 1985 Mar-Apr;7 (Suppl 1):S150–S156. doi: 10.1093/clinids/7.supplement_1.s150. [DOI] [PubMed] [Google Scholar]
- Fayolle C., Deriaud E., Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol. 1991 Dec 15;147(12):4069–4073. [PubMed] [Google Scholar]
- Fraser J. R., Cunningham A. L., Hayes K., Leach R., Lunt R. Rubella arthritis in adults. Isolation of virus, cytology and other aspects of the synovial reaction. Clin Exp Rheumatol. 1983 Oct-Dec;1(4):287–293. [PubMed] [Google Scholar]
- Giles R. C., Capra J. D. Structure, function, and genetics of human class II molecules. Adv Immunol. 1985;37:1–71. doi: 10.1016/s0065-2776(08)60337-5. [DOI] [PubMed] [Google Scholar]
- Giles R. C., Nunez G., Hurley C. K., Nunez-Roldan A., Winchester R., Stastny P., Capra J. D. Structural analysis of a human I-A homologue using a monoclonal antibody that recognizes an MB3-like specificity. J Exp Med. 1983 May 1;157(5):1461–1470. doi: 10.1084/jem.157.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Y., Dorsett P. H. Rubella virus antigens: localization of epitopes involved in hemagglutination and neutralization by using monoclonal antibodies. J Virol. 1986 Mar;57(3):893–898. doi: 10.1128/jvi.57.3.893-898.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner H., Schliephake A., Winter J., Zimprich F., Lassmann H., Sedgwick J., Siddell S., Wege H. Nucleocapsid or spike protein-specific CD4+ T lymphocytes protect against coronavirus-induced encephalomyelitis in the absence of CD8+ T cells. J Immunol. 1991 Oct 1;147(7):2317–2323. [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Littaua R. A., Oldstone M. B., Takeda A., Ennis F. A. A CD4+ cytotoxic T-lymphocyte clone to a conserved epitope on human immunodeficiency virus type 1 p24: cytotoxic activity and secretion of interleukin-2 and interleukin-6. J Virol. 1992 Jan;66(1):608–611. doi: 10.1128/jvi.66.1.608-611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit H., Spouge J. L., Cornette J. L., Cease K. B., Delisi C., Berzofsky J. A. Prediction of immunodominant helper T cell antigenic sites from the primary sequence. J Immunol. 1987 Apr 1;138(7):2213–2229. [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Thornton G. B., Hughes J. L. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987 Oct 8;329(6139):547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- Milich D. R. Synthetic T and B cell recognition sites: implications for vaccine development. Adv Immunol. 1989;45:195–282. doi: 10.1016/s0065-2776(08)60694-x. [DOI] [PubMed] [Google Scholar]
- Oker-Blom C., Kalkkinen N., Käriäinen L., Pettersson R. F. Rubella virus contains one capsid protein and three envelope glycoproteins, E1, E2a, and E2b. J Virol. 1983 Jun;46(3):964–973. doi: 10.1128/jvi.46.3.964-973.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou D., Chong P., Tripet B., Gillam S. Analysis of T- and B-cell epitopes of capsid protein of rubella virus by using synthetic peptides. J Virol. 1992 Mar;66(3):1674–1681. doi: 10.1128/jvi.66.3.1674-1681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Steinman R. M., Hair L. S., Luban J., Witmer M. D., Koide S., Cohn Z. A. Specific antimononuclear phagocyte monoclonal antibodies. Application to the purification of dendritic cells and the tissue localization of macrophages. J Exp Med. 1983 Jul 1;158(1):126–145. doi: 10.1084/jem.158.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky J. S., McCarthy M., Allen-Cannady O., Moore W. T., Jin R., Cao S. N., Lovett A., Simmons D. Monoclonal antibody-defined epitope map of expressed rubella virus protein domains. J Virol. 1991 Aug;65(8):3986–3994. doi: 10.1128/jvi.65.8.3986-3994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Binnendijk R. S., Poelen M. C., Kuijpers K. C., Osterhaus A. D., Uytdehaag F. G. The predominance of CD8+ T cells after infection with measles virus suggests a role for CD8+ class I MHC-restricted cytotoxic T lymphocytes (CTL) in recovery from measles. Clonal analyses of human CD8+ class I MHC-restricted CTL. J Immunol. 1990 Mar 15;144(6):2394–2399. [PubMed] [Google Scholar]



