Abstract
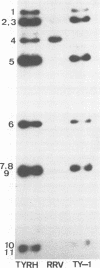
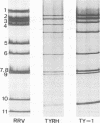
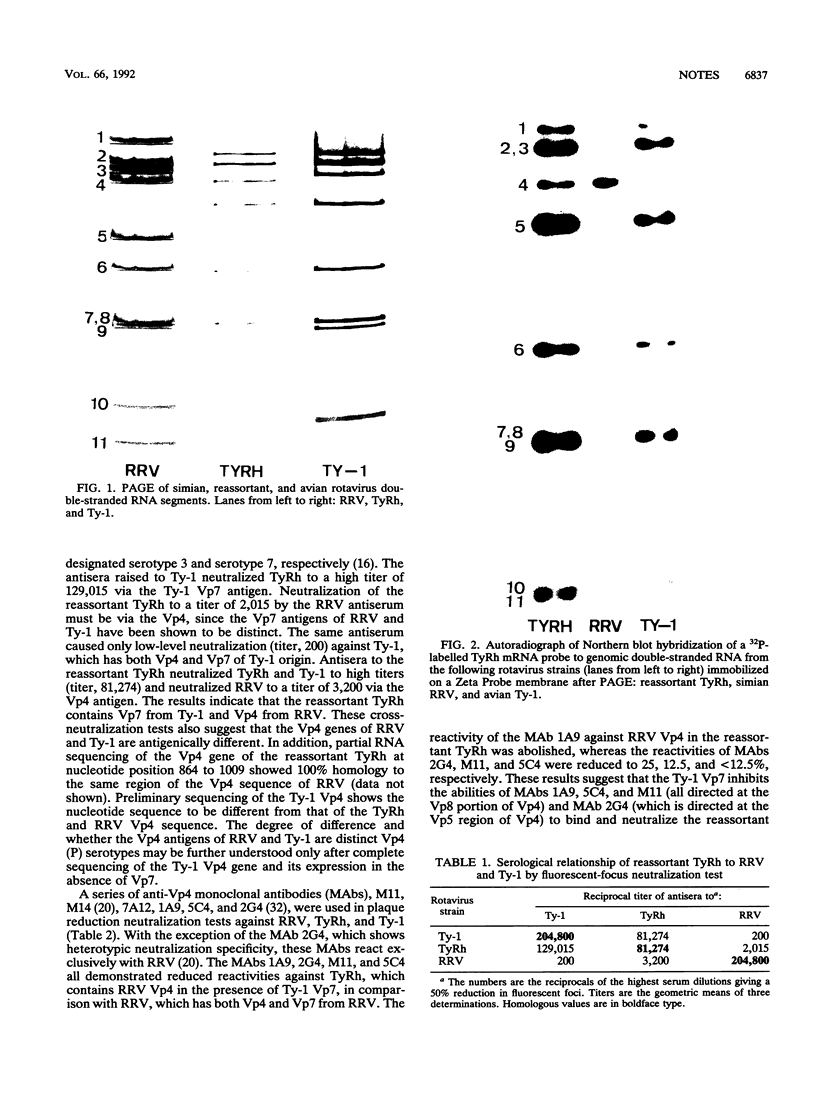
A reassortant, TyRh, was isolated after coinfection of MA104 cells with avian Ty-1 and simian RRV rotaviruses. Hybridization and serological studies showed that the reassortant's 4th gene, which encodes Vp4, was derived from the simian RRV rotavirus parent, whereas the remaining 10 genes were derived from the avian Ty-1 rotavirus parent.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastardo J. W., McKimm-Breschkin J. L., Sonza S., Mercer L. D., Holmes I. H. Preparation and characterization of antisera to electrophoretically purified SA11 virus polypeptides. Infect Immun. 1981 Dec;34(3):641–647. doi: 10.1128/iai.34.3.641-647.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin D. K., Knudson D. L. Sequence relatedness of Palyam virus genes to cognates of the Palyam serogroup viruses by RNA-RNA blot hybridization. Virology. 1985 May;143(1):55–62. doi: 10.1016/0042-6822(85)90096-0. [DOI] [PubMed] [Google Scholar]
- Chen D. Y., Estes M. K., Ramig R. F. Specific interactions between rotavirus outer capsid proteins VP4 and VP7 determine expression of a cross-reactive, neutralizing VP4-specific epitope. J Virol. 1992 Jan;66(1):432–439. doi: 10.1128/jvi.66.1.432-439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Gene-coding assignments of rotavirus double-stranded RNA segments 10 and 11. J Virol. 1981 Jun;38(3):1099–1103. doi: 10.1128/jvi.38.3.1099-1103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Midthun K., Hoshino Y., Green K., Gorziglia M., Kapikian A. Z., Chanock R. M. Conservation of the fourth gene among rotaviruses recovered from asymptomatic newborn infants and its possible role in attenuation. J Virol. 1986 Dec;60(3):972–979. doi: 10.1128/jvi.60.3.972-979.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombold J. L., Ramig R. F. Analysis of reassortment of genome segments in mice mixedly infected with rotaviruses SA11 and RRV. J Virol. 1986 Jan;57(1):110–116. doi: 10.1128/jvi.57.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Flores J., Kalica A. R., Wyatt R. G., Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983 Feb;64(Pt 2):313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Kalica A. R., Wyatt R. G., Jones R. W., Kapikian A. Z., Chanock R. M. Rescue of noncultivatable human rotavirus by gene reassortment during mixed infection with ts mutants of a cultivatable bovine rotavirus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):420–424. doi: 10.1073/pnas.78.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Wyatt R. G., Kapikian A. Z., Kalica A. R., Flores J., Jones R. Rescue and serotypic characterization of noncultivable human rotavirus by gene reassortment. Infect Immun. 1982 Jul;37(1):104–109. doi: 10.1128/iai.37.1.104-109.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Saif L. J., Sereno M. M., Chanock R. M., Kapikian A. Z. Infection immunity of piglets to either VP3 or VP7 outer capsid protein confers resistance to challenge with a virulent rotavirus bearing the corresponding antigen. J Virol. 1988 Mar;62(3):744–748. doi: 10.1128/jvi.62.3.744-748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Chanock R. M., Kapikian A. Z. Analysis by plaque reduction neutralization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J Clin Microbiol. 1987 Feb;25(2):290–294. doi: 10.1128/jcm.25.2.290-294.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Greenberg H. B., Wyatt R. G., Flores J., Sereno M. M., Kapikian A. Z., Chanock R. M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981 Jul 30;112(2):385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- López S., Arias C. F., Bell J. R., Strauss J. H., Espejo R. T. Primary structure of the cleavage site associated with trypsin enhancement of rotavirus SA11 infectivity. Virology. 1985 Jul 15;144(1):11–19. doi: 10.1016/0042-6822(85)90300-9. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Dang M. N., Greenberg H. B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci U S A. 1988 Feb;85(3):645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Kalica A. R., Kono R. Isolation of a recombinant between simian and bovine rotaviruses. J Gen Virol. 1980 May;48(1):253–256. doi: 10.1099/0022-1317-48-1-253. [DOI] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Hoshino Y., Kapikian A. Z., Chanock R. M. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J Clin Microbiol. 1986 Nov;24(5):822–826. doi: 10.1128/jcm.24.5.822-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Valdesuso J., Hoshino Y., Flores J., Kapikian A. Z., Chanock R. M. Analysis by RNA-RNA hybridization assay of intertypic rotaviruses suggests that gene reassortment occurs in vivo. J Clin Microbiol. 1987 Feb;25(2):295–300. doi: 10.1128/jcm.25.2.295-300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesha H. S., Holmes I. H. New porcine rotavirus serotype antigenically related to human rotavirus serotype 3. J Clin Microbiol. 1988 Feb;26(2):171–174. doi: 10.1128/jcm.26.2.171-174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Oyamada H., Nakagomi T. Use of alkaline northern blot hybridization for the identification of genetic relatedness of the fourth gene of rotaviruses. Mol Cell Probes. 1989 Sep;3(3):263–271. doi: 10.1016/0890-8508(89)90007-8. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Hoshino Y., Gorziglia M. Sequence of the VP7 gene of chicken rotavirus Ch2 strain of serotype 7 rotavirus. Virology. 1991 Dec;185(2):853–856. doi: 10.1016/0042-6822(91)90558-s. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Shaw R. D., Greenberg H. B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986 May;58(2):700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Wyatt R. G., Greenberg H. B., James W. D., Pittman A. L., Kalica A. R., Flores J., Chanock R. M., Kapikian A. Z. Definition of human rotavirus serotypes by plaque reduction assay. Infect Immun. 1982 Jul;37(1):110–115. doi: 10.1128/iai.37.1.110-115.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M., Dryden K. A., Olson N. H., Greenberg H. B., Baker T. S. Three-dimensional structure of rhesus rotavirus by cryoelectron microscopy and image reconstruction. J Cell Biol. 1990 Jun;110(6):2133–2144. doi: 10.1083/jcb.110.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]