Abstract
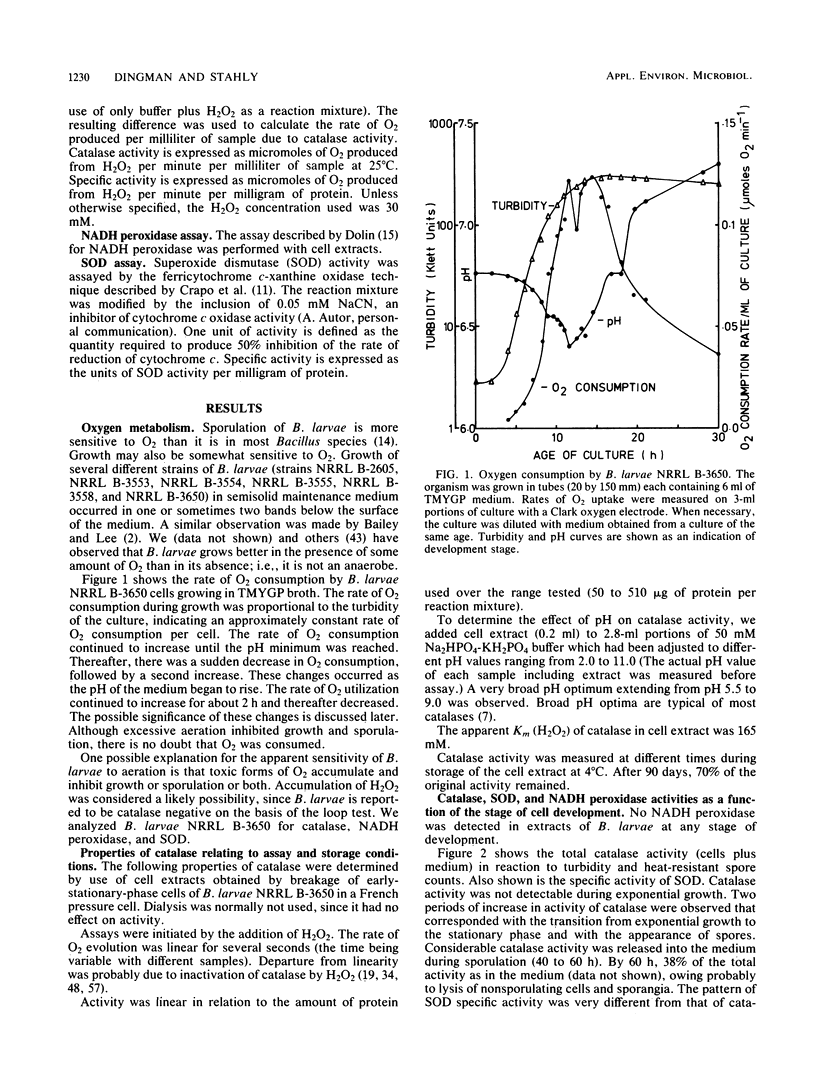
Sporulation of Bacillus larvae NRRL B-3650 occurred only at aeration rates lower than those used for cultivation of most Bacillus species. One possible explanation for the requirement for a low level of aeration in B. larvae is that toxic forms of oxygen such as H2O2 and superoxide are involved. The superoxide dismutase levels of strain B-3650 were similar to those of Bacillus subtilis 168 during sporulation, and no NADH peroxidase was present. Catalase activity was absent during exponential growth and first appeared near the start of the stationary phase. The catalase activity was 2,700 times less than that in B. subtilis 168 at the same stage of development. Therefore, the relative deficiency of catalase (and NADH peroxidase) might be the cause of the apparent O2 toxicity. It was postulated that B. larvae might accumulate H2O2 in the medium and exhibit more than normal sensitivity to H2O2. Experimental results did not verify either postulate, but the possibilities of intracellular accumulation of H2O2 and unusual sensitivity to endogenous H2O2 were not excluded. The catalase present in early-stationary-phase cells was soluble, heat labile, and inhibited by cyanide, azide, and hydroxylamine. An increase in catalase activity also occurred at the time of appearance of refractile spores in both B. larvae NRRL B-3650 and B. subtilis 168. The level of catalase activity in strain B-3650 was 5,400 times less than that in B. subtilis 168 at this stage. In B. larvae, this second increase occurred primarily within the developing endospore. The activity in spore extracts was particulate, heat stable, and inhibited by hydroxylamine but not by azide or cyanide. Synthesis of catalase in B. larvae was unaffected by H2O2, O2, or glucose.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACH J. A., SADOFF H. L. Aerobic sporulating bacteria. I. Glucose dehydrogenase of Bacillus cereus. J Bacteriol. 1962 Apr;83:699–707. doi: 10.1128/jb.83.4.699-707.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY L., LEE D. C. Bacillus larvae: its cultivation in vitro and its growth in vivo. J Gen Microbiol. 1962 Dec;29:711–717. doi: 10.1099/00221287-29-4-711. [DOI] [PubMed] [Google Scholar]
- Baillie A., Norris J. R. Antigen changes during spore formation in Bacillus cereus. J Bacteriol. 1964 May;87(5):1221–1226. doi: 10.1128/jb.87.5.1221-1226.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. Effect of pH upon the reaction kinetics of the enzyme-substrate compounds of catalase. J Biol Chem. 1952 Feb;194(2):471–481. [PubMed] [Google Scholar]
- CLAYTON R. K. Permeability barriers and the assay of catalase in intact cells. Biochim Biophys Acta. 1959 Nov;36:35–39. doi: 10.1016/0006-3002(59)90066-6. [DOI] [PubMed] [Google Scholar]
- Costilow R. N., Coulter W. H. Physiological studies of an oligosporogenous strain of Bacillus popilliae. Appl Microbiol. 1971 Dec;22(6):1076–1084. doi: 10.1128/am.22.6.1076-1084.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costilow R. N., Sylvester C. J., Pepper R. E. Production and stabilization of cells of Bacillus popilliae and Bacillus lentimorbus. Appl Microbiol. 1966 Mar;14(2):161–169. doi: 10.1128/am.14.2.161-169.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D., McCord J. M., Fridovich I. Preparation and assay of superoxide dismutases. Methods Enzymol. 1978;53:382–393. doi: 10.1016/s0076-6879(78)53044-9. [DOI] [PubMed] [Google Scholar]
- DOLIN M. I. The Streptococcus faecalis oxidases for reduced diphosphopyridine nucleotide. III. Isolation and properties of a flavin peroxidase for reduced diphosphopyridine nucleotide. J Biol Chem. 1957 Mar;225(1):557–573. [PubMed] [Google Scholar]
- Deisseroth A., Dounce A. L. Catalase: Physical and chemical properties, mechanism of catalysis, and physiological role. Physiol Rev. 1970 Jul;50(3):319–375. doi: 10.1152/physrev.1970.50.3.319. [DOI] [PubMed] [Google Scholar]
- Del Río L. A., Ortega M. G., López A. L., Gorgé J. L. A more sensitive modification of the catalase assay with the Clark oxygen electrode. Application to the kinetic study of the pea leaf enzyme. Anal Biochem. 1977 Jun;80(2):409–415. doi: 10.1016/0003-2697(77)90662-5. [DOI] [PubMed] [Google Scholar]
- Dingman D. W., Stahly D. P. Medium Promoting Sporulation of Bacillus larvae and Metabolism of Medium Components. Appl Environ Microbiol. 1983 Oct;46(4):860–869. doi: 10.1128/aem.46.4.860-869.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEW A. V., FRASER M. J., GILBY A. R. The intracellular catalase of Micrococcus lysodeikticus. Biochim Biophys Acta. 1957 May;24(2):306–314. doi: 10.1016/0006-3002(57)90199-3. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Ramaley R., Freese E. Location and properties of glucose dehydrogenase in sporulating cells and spores of Bacillus subtilis. J Bacteriol. 1977 Oct;132(1):282–293. doi: 10.1128/jb.132.1.282-293.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. B. A method for assay of catalase with the oxygen cathode. Anal Biochem. 1968 Sep;24(3):431–437. doi: 10.1016/0003-2697(68)90148-6. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON H. M., WITOSLAWSKI J. J., CAMPBELL E. H. REVERSIBLE DISRUPTION OF A WAVELENGTH DISCRIMINATION IN PIGEONS FOLLOWING ADMINISTRATION OF PHENIPRAZINE. Toxicol Appl Pharmacol. 1964 Nov;6:690–695. doi: 10.1016/0041-008x(64)90119-x. [DOI] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of catalase and peroxidase in Escherichia coli. J Biol Chem. 1978 Sep 25;253(18):6445–6420. [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. Catalase of the Lacto-bacillaceae. J Bacteriol. 1962 Apr;83:936–938. doi: 10.1128/jb.83.4.936-938.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON M. A., DELWICHE E. A. DISTRIBUTION AND CHARACTERISTICS OF THE CATALASES OF LACTOBACILLACEAE. J Bacteriol. 1965 Aug;90:347–351. doi: 10.1128/jb.90.2.347-351.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P., Suggett A. The catalse-hydrogen peroxide system. Kinetics of catalatic action at high substrate concentrations. Biochem J. 1968 Dec;110(4):617–620. doi: 10.1042/bj1100617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN J. G. The alteration of intracellular enzymes. I. Yeast catalase and the Euler effect. Exp Cell Res. 1955 Apr;8(2):305–328. doi: 10.1016/0014-4827(55)90143-9. [DOI] [PubMed] [Google Scholar]
- LAWRENCE N. L., HALVORSON H. O. Studies on the spores of aerobic bacteria. IV. A heat resistant catalase from spores of Bacillus terminalis. J Bacteriol. 1954 Sep;68(3):334–337. doi: 10.1128/jb.68.3.334-337.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton T. J., Doi R. H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971 May 25;246(10):3189–3195. [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- MYLROIE R. L., KATZNELSON H. Carbohydrate metabolism of Bacillus larvae. J Bacteriol. 1957 Aug;74(2):217–221. doi: 10.1128/jb.74.2.217-221.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORRIS J. R., BAILLIE A. IMMUNOLOGICAL SPECIFICITIES OF SPORE AND VEGETATIVE CELL CATALASES OF BACILLUS CEREUS. J Bacteriol. 1964 Jul;88:264–265. doi: 10.1128/jb.88.1.264-265.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P. The reaction of azide with catalase and their significance. Biochem J. 1964 Feb;90(2):331–343. doi: 10.1042/bj0900331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEPPER R. E., COSTILOW R. N. ELECTRON TRANSPORT IN BACILLUS POPILLIAE. J Bacteriol. 1965 Feb;89:271–276. doi: 10.1128/jb.89.2.271-276.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H. E., Loewen P. C. Induction of catalase in Escherichia coli by ascorbic acid involves hydrogen peroxide. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1039–1046. doi: 10.1016/0006-291x(81)91928-8. [DOI] [PubMed] [Google Scholar]
- Rorth M., Jensen P. K. Determination of catalase activity by means of the Clark oxygen electrode. Biochim Biophys Acta. 1967 May 16;139(1):171–173. doi: 10.1016/0005-2744(67)90124-6. [DOI] [PubMed] [Google Scholar]
- Steinkraus K. H., Tashiro H. Milky disease bacteria. Appl Microbiol. 1967 Mar;15(2):325–333. doi: 10.1128/am.15.2.325-333.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber H., Freese E. Sporulation properties of cytochrome a-deficient mutants of Bacillus subtilis. J Bacteriol. 1974 Dec;120(3):1004–1011. doi: 10.1128/jb.120.3.1004-1011.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters J. R., Stahly D. P. Modification of the valve of the French pressure cell. Appl Microbiol. 1968 Oct;16(10):1605–1605. doi: 10.1128/am.16.10.1605-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshpe-Purer Y., Henis Y. Factors affecting catalase level and sensitivity to hydrogen peroxide in Escherichia coli. Appl Environ Microbiol. 1976 Oct;32(4):465–469. doi: 10.1128/aem.32.4.465-469.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



