Abstract
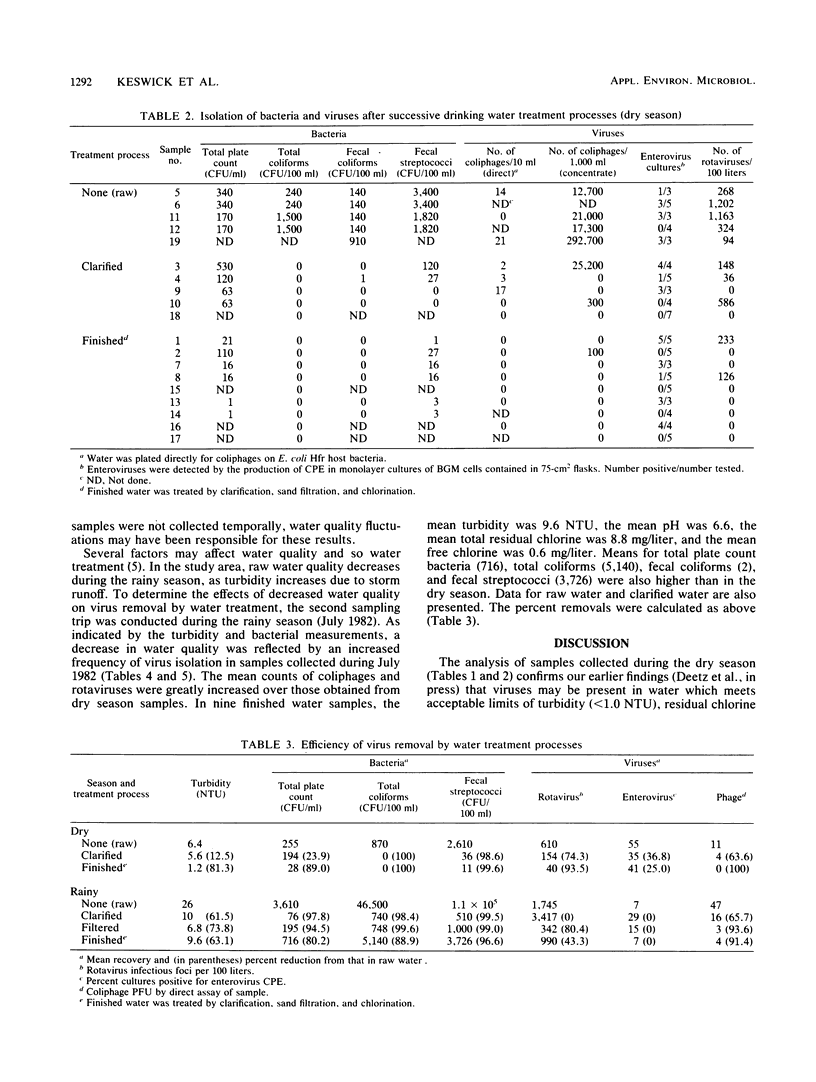
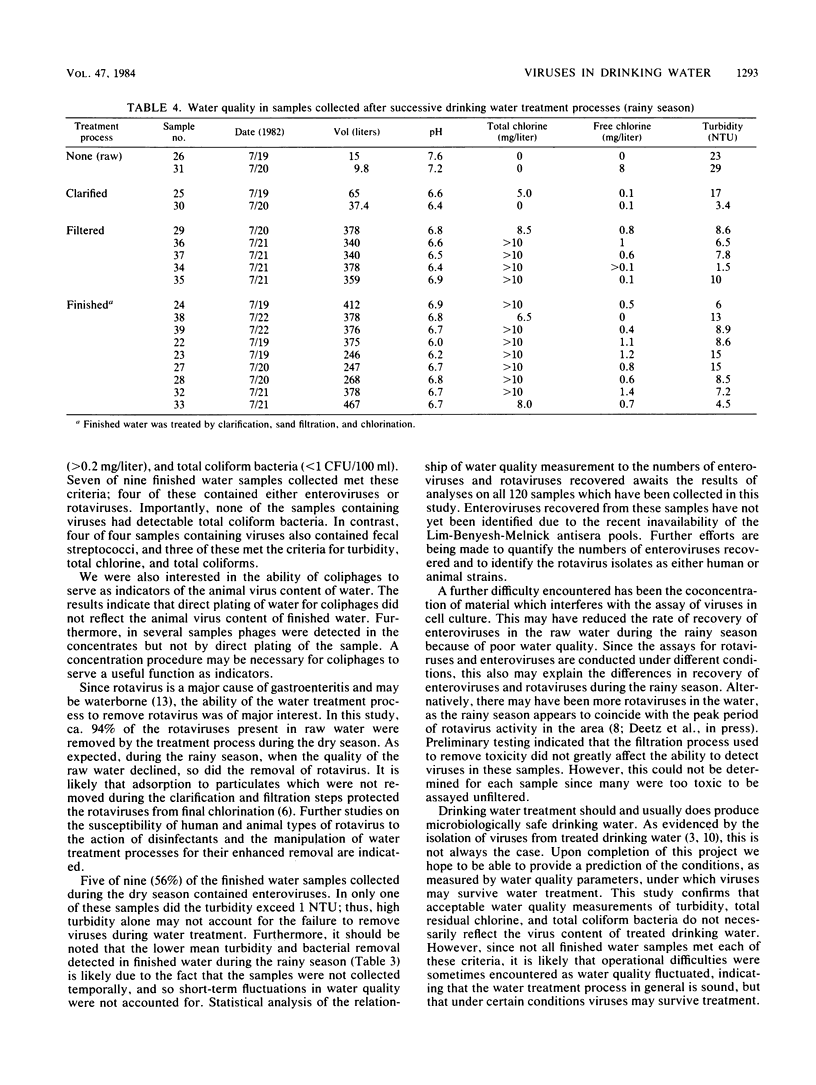
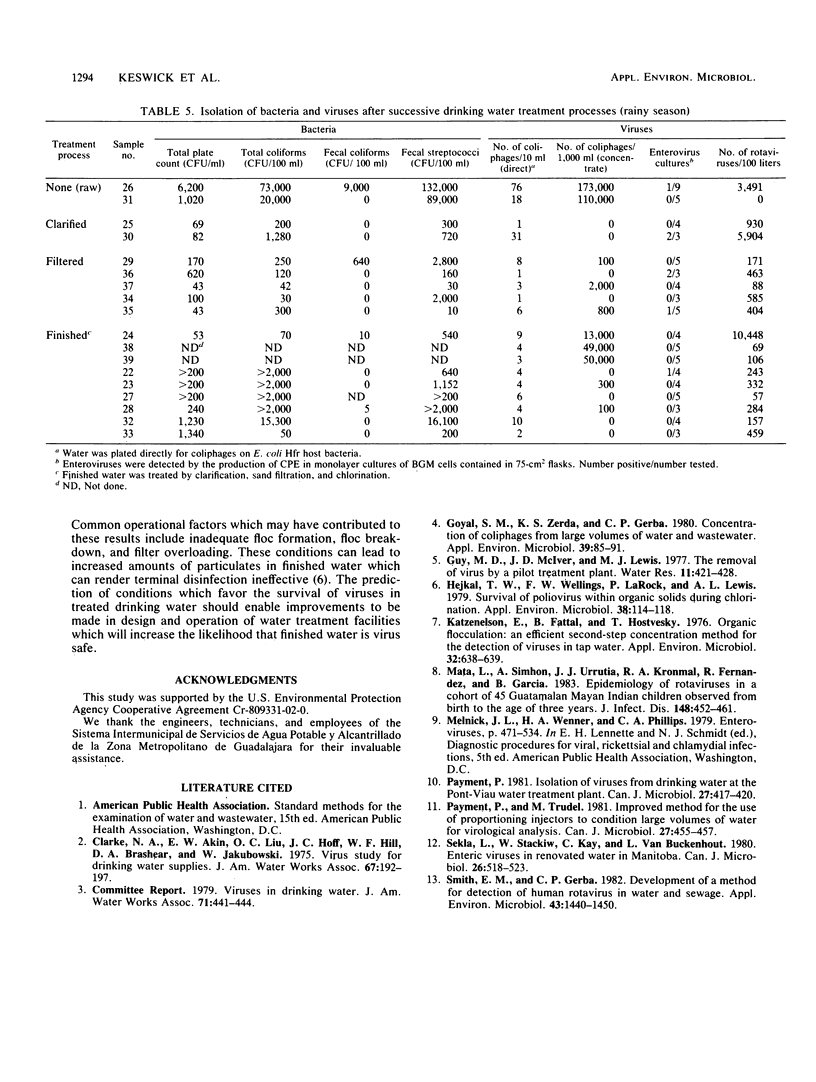
The occurrence of viruses in conventionally treated drinking water derived from a heavily polluted source was evaluated by collecting and analyzing 38 large-volume (65- to 756-liter) samples of water from a 9 m3/s (205 X 10(6) gallons [776 X 10(6) liters] per day) water treatment plant. Samples of raw, clarified, filtered, and chlorinated finished water were concentrated by using the filter adsorption-elution technique. Of 23 samples of finished water, 19 (83%) contained viruses. None of the nine finished water samples collected during the dry season contained detectable total coliform bacteria. Seven of nine finished water samples collected during the dry season met turbidity, total coliform bacteria, and total residual chlorine standards. Of these, four contained virus. During the dry season the percent removals were 25 to 93% for enteric viruses, 89 to 100% for bacteria, and 81% for turbidity. During the rainy season the percent removals were 0 to 43% for enteric viruses, 80 to 96% for bacteria, and 63% for turbidity. None of the 14 finished water samples collected during the rainy season met turbidity standards, and all contained rotaviruses or enteroviruses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goyal S. M., Zerda K. S., Gerba C. P. Concentration of coliphages from large volumes of water and wastewater. Appl Environ Microbiol. 1980 Jan;39(1):85–91. doi: 10.1128/aem.39.1.85-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejkal T. W., Wellings F. M., LaRock P. A., Lewis A. L. Survival of poliovirus within organic solids during chlorination. Appl Environ Microbiol. 1979 Jul;38(1):114–118. doi: 10.1128/aem.38.1.114-118.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenelson E., Fattal B., Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976 Oct;32(4):638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata L., Simhon A., Urrutia J. J., Kronmal R. A., Fernández R., García B. Epidemiology of rotaviruses in a cohort of 45 Guatamalan Mayan Indian children observed from birth to the age of three years. J Infect Dis. 1983 Sep;148(3):452–461. doi: 10.1093/infdis/148.3.452. [DOI] [PubMed] [Google Scholar]
- Payment P. Isolation of viruses from drinking water at the Point-Viau water treatment plant. Can J Microbiol. 1981 Apr;27(4):417–420. doi: 10.1139/m81-063. [DOI] [PubMed] [Google Scholar]
- Payment P., Trudel M. Improved method for the use of proportioning injectors to condition large volumes of water for virological analysis. Can J Microbiol. 1981 Apr;27(4):455–457. doi: 10.1139/m81-069. [DOI] [PubMed] [Google Scholar]
- Sekla L., Stackiw W., Kay C., VanBuckenhout L. Enteric viruses in renovated water in Manitoba. Can J Microbiol. 1980 Apr;26(4):518–523. doi: 10.1139/m80-087. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Gerba C. P. Development of a method for detection of human rotavirus in water and sewage. Appl Environ Microbiol. 1982 Jun;43(6):1440–1450. doi: 10.1128/aem.43.6.1440-1450.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]



