Abstract
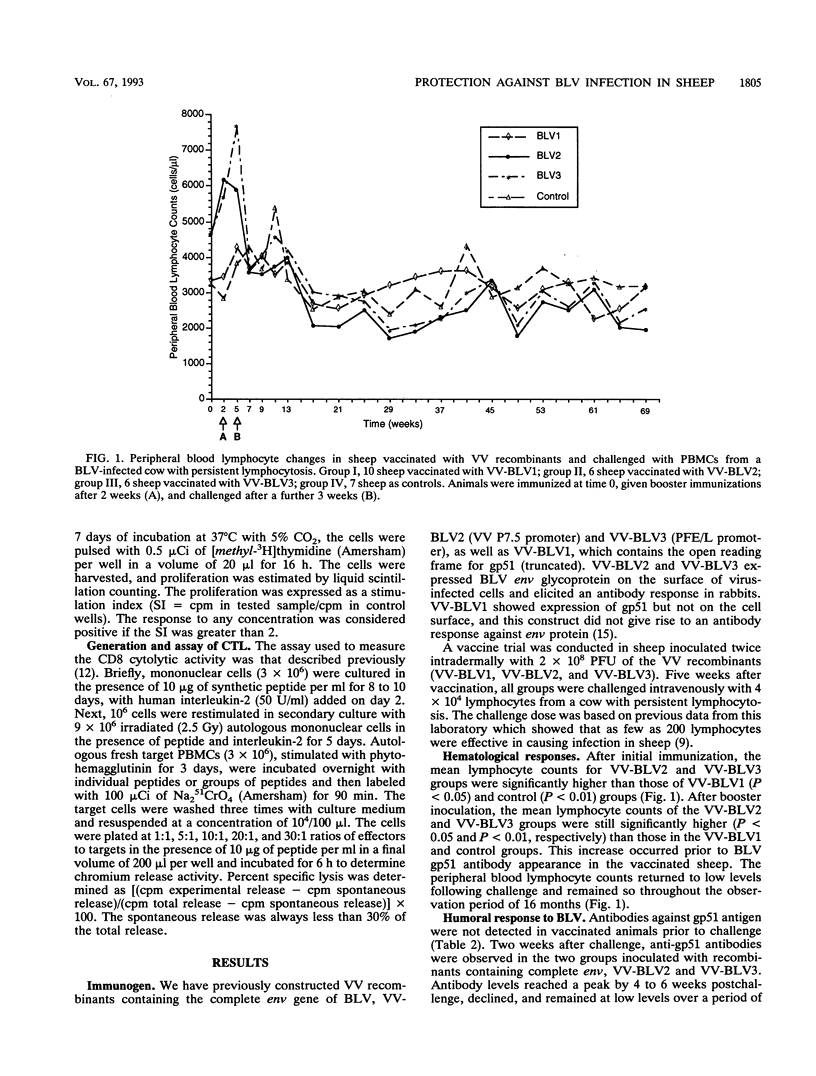
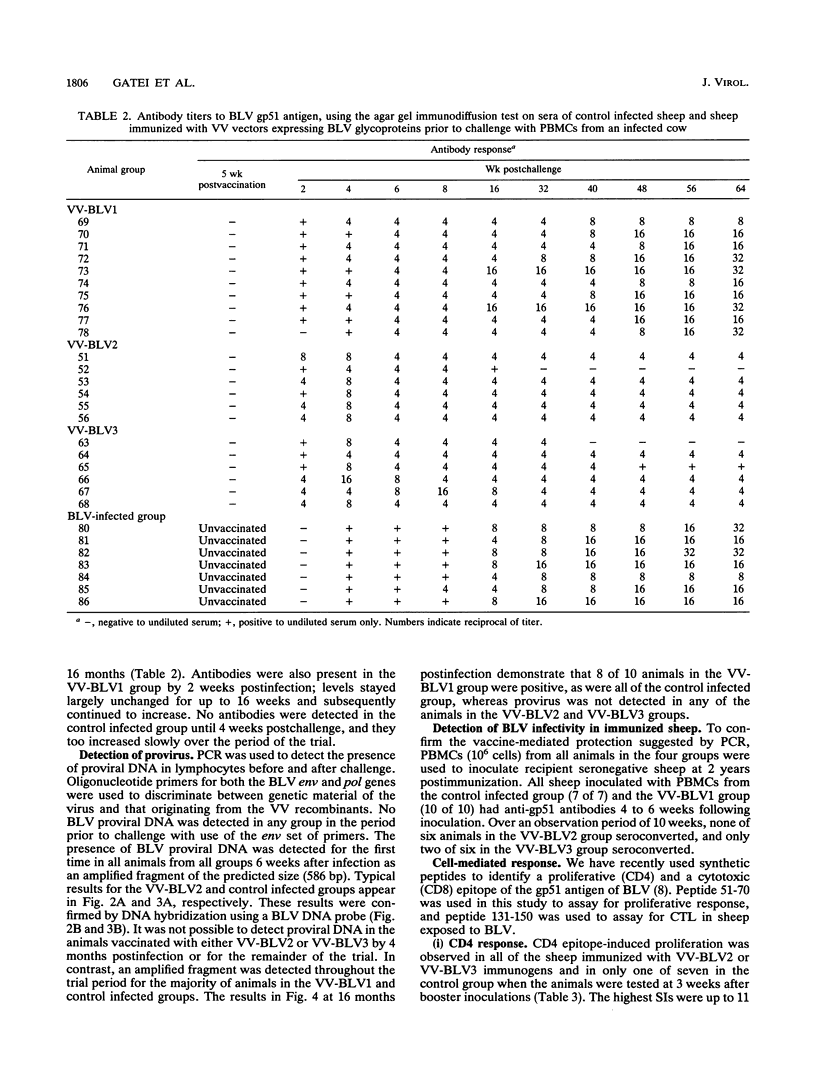
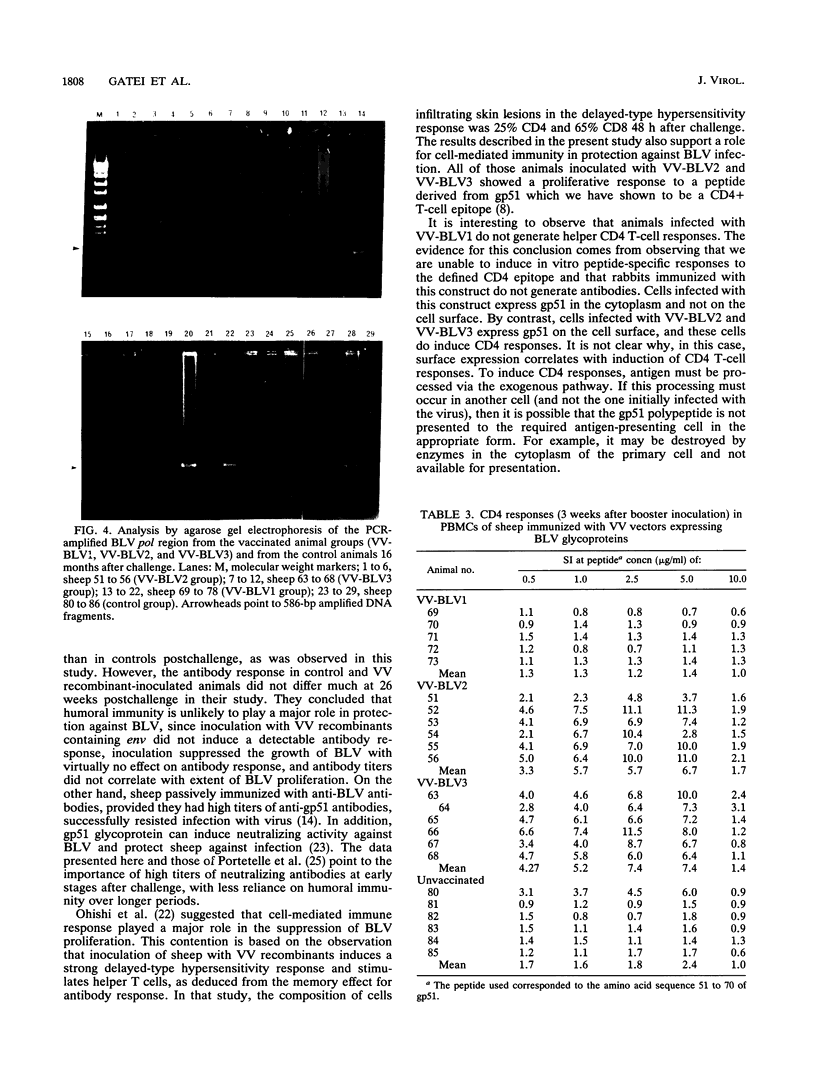
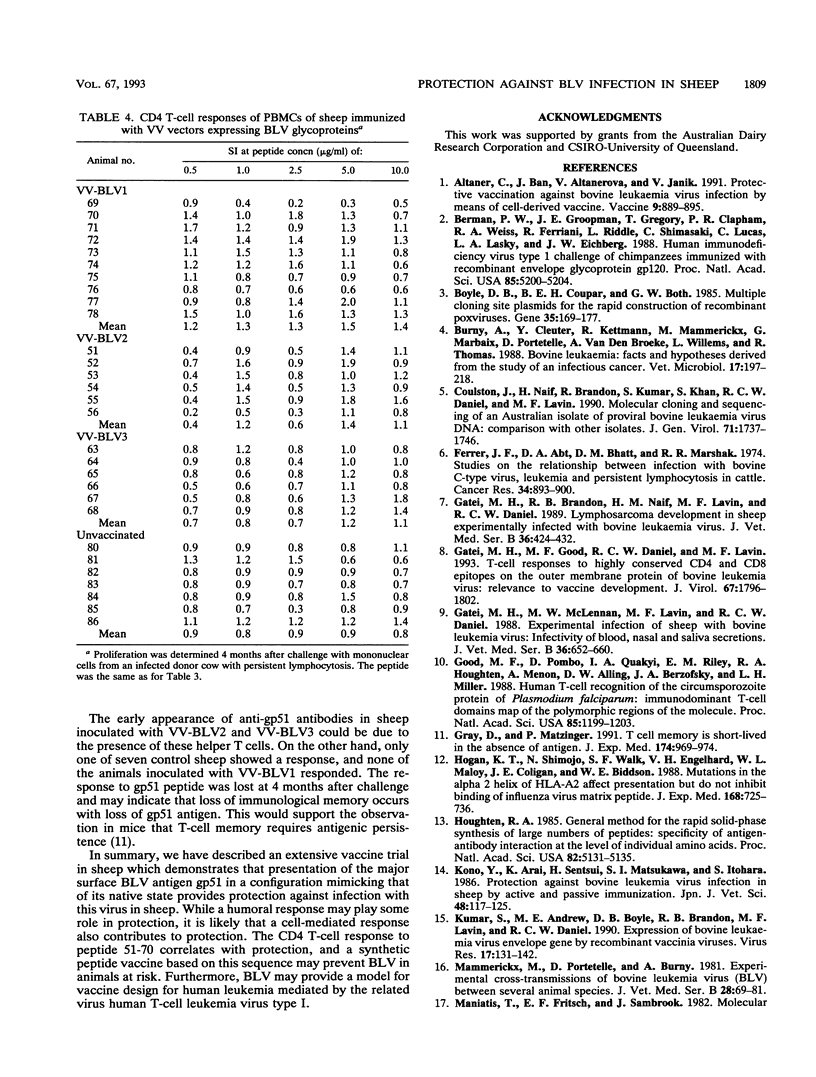
We have previously constructed vaccinia virus (VV) recombinants containing a complete or truncated envelope (env) gene of bovine leukemia virus (BLV). Only recombinants carrying the complete env gene (VV-BLV2 and VV-BLV3) expressed env glycoprotein on the surface of virus-infected cells and produced an antibody response in rabbits. In the present study, these VV recombinants were used to immunize sheep prior to challenge with BLV-infected peripheral blood mononuclear cells. Both humoral and cell-mediated immunity were monitored in infected animals. Sheep inoculated with recombinants containing the complete env gene showed a CD4 response to a defined epitope of gp51, but this response was absent 4 months postchallenge. Anti-gp51 antibodies appeared in animals inoculated with complete env 2 weeks after challenge, reached a peak at 4 weeks, and subsequently declined over 16 months. No CD4 response was recorded in animals inoculated with recombinants containing truncated env gene (VV-BLV1). BLV-infected control animals and those animals receiving VV-BLV1 were slower to develop antibodies postchallenge, and the titers of anti-gp51 antibodies continued to increase over 16 months. Proviral DNA was detected by the polymerase chain reaction in the four groups at 6 weeks after challenge. However, it could not be detected 4 months postinfection in the VV groups inoculated with complete env. Provirus was present in the VV-BLV1 and control groups over the 16-month trial period. These results demonstrate that vaccination with VV recombinants containing the complete env gene of BLV protects sheep against infection and that protection correlated with a CD4 T-cell response to a defined epitope.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altaner C., Ban J., Altanerova V., Janik V. Protective vaccination against bovine leukaemia virus infection by means of cell-derived vaccine. Vaccine. 1991 Dec;9(12):889–895. doi: 10.1016/0264-410x(91)90009-u. [DOI] [PubMed] [Google Scholar]
- Berman P. W., Groopman J. E., Gregory T., Clapham P. R., Weiss R. A., Ferriani R., Riddle L., Shimasaki C., Lucas C., Lasky L. A. Human immunodeficiency virus type 1 challenge of chimpanzees immunized with recombinant envelope glycoprotein gp120. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5200–5204. doi: 10.1073/pnas.85.14.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle D. B., Coupar B. E., Both G. W. Multiple-cloning-site plasmids for the rapid construction of recombinant poxviruses. Gene. 1985;35(1-2):169–177. doi: 10.1016/0378-1119(85)90169-6. [DOI] [PubMed] [Google Scholar]
- Burny A., Cleuter Y., Kettmann R., Mammerickx M., Marbaix G., Portetelle D., van den Broeke A., Willems L., Thomas R. Bovine leukaemia: facts and hypotheses derived from the study of an infectious cancer. Vet Microbiol. 1988 Jul;17(3):197–218. doi: 10.1016/0378-1135(88)90066-1. [DOI] [PubMed] [Google Scholar]
- Coulston J., Naif H., Brandon R., Kumar S., Khan S., Daniel R. C., Lavin M. F. Molecular cloning and sequencing of an Australian isolate of proviral bovine leukaemia virus DNA: comparison with other isolates. J Gen Virol. 1990 Aug;71(Pt 8):1737–1746. doi: 10.1099/0022-1317-71-8-1737. [DOI] [PubMed] [Google Scholar]
- Ferrer J. F., Abt D. A., Bhatt D. M., Marshak R. R. Studies on the relationship between infection with bovine C-type virus, leukemia, and persistent lymphocytosis in cattle. Cancer Res. 1974 Apr;34(4):893–900. [PubMed] [Google Scholar]
- Gatei M. H., Brandon R., Naif H. M., Lavin M. F., Daniel R. C. Lymphosarcoma development in sheep experimentally infected with bovine leukaemia virus. Zentralbl Veterinarmed B. 1989 Aug;36(6):424–432. doi: 10.1111/j.1439-0450.1989.tb00624.x. [DOI] [PubMed] [Google Scholar]
- Gatei M. H., Good M. F., Daniel R. C., Lavin M. F. T-cell responses to highly conserved CD4 and CD8 epitopes on the outer membrane protein of bovine leukemia virus: relevance to vaccine development. J Virol. 1993 Apr;67(4):1796–1802. doi: 10.1128/jvi.67.4.1796-1802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatei M. H., McLennan M. W., Lavin M. F., Daniel R. C. Experimental infection of sheep with bovine leukemia virus: infectivity of blood, nasal and saliva secretions. Zentralbl Veterinarmed B. 1989 Nov;36(9):652–660. doi: 10.1111/j.1439-0450.1989.tb00658.x. [DOI] [PubMed] [Google Scholar]
- Good M. F., Pombo D., Quakyi I. A., Riley E. M., Houghten R. A., Menon A., Alling D. W., Berzofsky J. A., Miller L. H. Human T-cell recognition of the circumsporozoite protein of Plasmodium falciparum: immunodominant T-cell domains map to the polymorphic regions of the molecule. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1199–1203. doi: 10.1073/pnas.85.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D., Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991 Nov 1;174(5):969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan K. T., Shimojo N., Walk S. F., Engelhard V. H., Maloy W. L., Coligan J. E., Biddison W. E. Mutations in the alpha 2 helix of HLA-A2 affect presentation but do not inhibit binding of influenza virus matrix peptide. J Exp Med. 1988 Aug 1;168(2):725–736. doi: 10.1084/jem.168.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghten R. A. General method for the rapid solid-phase synthesis of large numbers of peptides: specificity of antigen-antibody interaction at the level of individual amino acids. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5131–5135. doi: 10.1073/pnas.82.15.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono Y., Arai K., Sentsui H., Matsukawa S., Itohara S. Protection against bovine leukemia virus infection in sheep by active and passive immunization. Nihon Juigaku Zasshi. 1986 Feb;48(1):117–125. doi: 10.1292/jvms1939.48.117. [DOI] [PubMed] [Google Scholar]
- Kumar S., Andrew M. E., Boyle D. B., Brandon R. B., Lavin M. F., Daniel R. C. Expression of bovine leukaemia virus envelope gene by recombinant vaccinia viruses. Virus Res. 1990 Oct;17(2):131–142. doi: 10.1016/0168-1702(90)90074-l. [DOI] [PubMed] [Google Scholar]
- Mammerickx M., Portetelle D., Burny A. Experimental cross-transmissions of bovine leukemia virus (BLV) between several animal species. Zentralbl Veterinarmed B. 1981;28(1):69–81. doi: 10.1111/j.1439-0450.1981.tb01740.x. [DOI] [PubMed] [Google Scholar]
- Miller J. M., Miller L. D., Olson C., Gillette K. G. Virus-like particles in phytohemagglutinin-stimulated lymphocyte cultures with reference to bovine lymphosarcoma. J Natl Cancer Inst. 1969 Dec;43(6):1297–1305. [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naif H. M., Brandon R. B., Daniel R. C., Lavin M. F. Bovine leukaemia proviral DNA detection in cattle using the polymerase chain reaction. Vet Microbiol. 1990 Nov;25(2-3):117–129. doi: 10.1016/0378-1135(90)90071-3. [DOI] [PubMed] [Google Scholar]
- Ohishi K., Maruyama T., Shida H., Nishimaki J., Miki K., Sagata N., Ikawa Y., Sugimoto M. Immunogenicity of a recombinant vaccinia virus expressing envelope a glycoprotein of bovine leukaemia virus. Vaccine. 1988 Oct;6(5):428–432. doi: 10.1016/0264-410x(88)90144-2. [DOI] [PubMed] [Google Scholar]
- Ohishi K., Suzuki H., Yamamoto T., Maruyama T., Miki K., Ikawa Y., Numakunai S., Okada K., Ohshima K., Sugimoto M. Protective immunity against bovine leukaemia virus (BLV) induced in carrier sheep by inoculation with a vaccinia virus-BLV env recombinant: association with cell-mediated immunity. J Gen Virol. 1991 Aug;72(Pt 8):1887–1892. doi: 10.1099/0022-1317-72-8-1887. [DOI] [PubMed] [Google Scholar]
- Onuma M., Hodatsu T., Yamamoto S., Higashihara M., Masu S., Mikami T., Izawa H. Protection by vaccination against bovine leukemia virus infection in sheep. Am J Vet Res. 1984 Jun;45(6):1212–1215. [PubMed] [Google Scholar]
- Portetelle D., Bruck C., Burny A., Dekegel D., Mammerickx M., Urbain J. Detection of complement-dependent lytic antibodies in sera from bovine leukemia virus-infected animals. Ann Rech Vet. 1978;9(4):667–674. [PubMed] [Google Scholar]
- Portetelle D., Limbach K., Burny A., Mammerickx M., Desmettre P., Riviere M., Zavada J., Paoletti E. Recombinant vaccinia virus expression of the bovine leukaemia virus envelope gene and protection of immunized sheep against infection. Vaccine. 1991 Mar;9(3):194–200. doi: 10.1016/0264-410x(91)90153-w. [DOI] [PubMed] [Google Scholar]
- Shafferman A., Jahrling P. B., Benveniste R. E., Lewis M. G., Phipps T. J., Eden-McCutchan F., Sadoff J., Eddy G. A., Burke D. S. Protection of macaques with a simian immunodeficiency virus envelope peptide vaccine based on conserved human immunodeficiency virus type 1 sequences. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7126–7130. doi: 10.1073/pnas.88.16.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]