Abstract
C1 neurotoxin of Clostridium botulinum strains C-Stockholm (C-ST), C beta-Yoichi, C-468, CD6F, and C-CB19 and type D toxin of strains D-1873 and D-CB16 were purified by gel filtration, ion exchange, and affinity chromatographies. The purified toxins had di-chain structure made of heavy and light chains. The toxins of C beta-Yoichi, C-468, CD6F, and C-CB19 reacted with anti-C-ST heavy chain and anti-C-ST light chain in immunodiffusion tests and enzyme-linked immunosorbent assay, whereas D-CB16 toxin reacted with anti-D-1873 heavy chain and anti-D-1873 light chain. However, C-6813 toxin reacted with anti-D-1873 heavy chain and anti-C-ST light chain but not with anti-C-ST heavy chain or anti-D-1873 light chain immunoglobulin G. These results indicate common antigens in the heavy chains of C-6813 and D-1873 toxins and in the light chains of C-6813 and C-ST toxins. Further, they provide evidence for heterogeneity within type C1 toxin subunits.
Full text
PDF

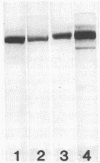
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boroff D. A., Fleck U. Statistical analysis of a rapid in vivo method for the titration of the toxin of Clostridium botulinum. J Bacteriol. 1966 Nov;92(5):1580–1581. doi: 10.1128/jb.92.5.1580-1581.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta B. R., Sugiyama H. A common subunit structure in Clostridium botulinum type A, B and E toxins. Biochem Biophys Res Commun. 1972 Jul 11;48(1):108–112. doi: 10.1016/0006-291x(72)90350-6. [DOI] [PubMed] [Google Scholar]
- Inoue K., Iida H. Phage-conversion of toxigenicity in Clostridium botulinum types C and D. Jpn J Med Sci Biol. 1971 Feb;24(1):53–56. [PubMed] [Google Scholar]
- Jansen B. C. The toxic antigenic factors produced by Clostridium botulinum types C and D. Onderstepoort J Vet Res. 1971 Jun;38(2):93–98. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Notermans S., Hagenaars A. M., Kozaki S. The enzyme-linked immunosorbent assay (ELISA) for the detection and determination of Clostridium botulinum toxins A, B, and E. Methods Enzymol. 1982;84:223–238. doi: 10.1016/0076-6879(82)84020-2. [DOI] [PubMed] [Google Scholar]
- Oguma K., Agui T., Syuto B., Kimura K., Iida H., Kubo S. Four different monoclonal antibodies against type C1 toxin of Clostridium botulinum. Infect Immun. 1982 Oct;38(1):14–20. doi: 10.1128/iai.38.1.14-20.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Syuto B., Agui T., Iida H., Kubo S. Homogeneity and heterogeneity of toxins produced by Clostridium botulinum type C and D strains. Infect Immun. 1981 Nov;34(2):382–388. doi: 10.1128/iai.34.2.382-388.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma K., Syuto B., Iida H., Kubo S. Antigenic similarity of toxins produced by Clostridium botulinum type C and D strains. Infect Immun. 1980 Dec;30(3):656–660. doi: 10.1128/iai.30.3.656-660.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syuto B., Kubo S. Isolation and molecular size of Clostridium botulinum type C toxin. Appl Environ Microbiol. 1977 Feb;33(2):400–405. doi: 10.1128/aem.33.2.400-405.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syuto B., Kubo S. Separation and characterization of heavy and light chains from Clostridium botulinum type C toxin and their reconstitution. J Biol Chem. 1981 Apr 25;256(8):3712–3717. [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]