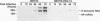Abstract
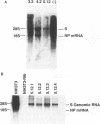
Ribozymes, RNA molecules which cleave RNA in a sequence-specific manner, are a promising tool in the development of specific antiviral therapies. The viruses most susceptible to ribozymes may be those in which all aspects of the viral life cycle depend on RNA, with no DNA intermediate. Consequently, we have chosen as a model one such virus, the arenavirus lymphocytic choriomeningitis virus (LCMV), and have previously reported the design of specific anti-LCMV ribozymes (Z. Xing and J. L. Whitton, J. Virol. 66:1361-1369, 1992). Here we describe the establishment of several cell lines, each stably expressing an antiarenaviral ribozyme of different specificity. Expression of a single ribozyme leads to a reduction in LCMV RNA levels, and stimulation of ribozyme transcription amplifies the effect. Target site selection may be an important determinant of antiviral effectiveness, since the extent of the antiviral effect, measured by assay of viral RNA, varies with the specificity of the antiviral ribozyme expressed. Furthermore, infectious virus production is reduced approximately 100-fold. This effect is LCMV specific, as yield of a related arenavirus is not similarly curtailed. We are currently investigating the mechanism underlying the ribozyme-mediated antiviral effect.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Ikeuchi T., Sun D., Sarin P. S., Konopka A., Maizel J., Zamecnik P. C. Inhibition of human immunodeficiency virus in early infected and chronically infected cells by antisense oligodeoxynucleotides and their phosphorothioate analogues. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7790–7794. doi: 10.1073/pnas.86.20.7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. J., Cullimore J. V. Selective cleavage of closely-related mRNAs by synthetic ribozymes. Nucleic Acids Res. 1992 Feb 25;20(4):831–837. doi: 10.1093/nar/20.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron K. K. Ganciclovir-resistant human cytomegalovirus clinical isolates; resistance mechanisms and in vitro susceptibility to antiviral agents. Transplant Proc. 1991 Jun;23(3 Suppl 3):162–167. [PubMed] [Google Scholar]
- Bishop D. H., Auperin D. D. Arenavirus gene structure and organization. Curr Top Microbiol Immunol. 1987;133:5–17. doi: 10.1007/978-3-642-71683-6_2. [DOI] [PubMed] [Google Scholar]
- Blum H. E., Galun E., von Weizsäcker F., Wands J. R. Inhibition of hepatitis B virus by antisense oligodeoxynucleotides. Lancet. 1991 May 18;337(8751):1230–1230. doi: 10.1016/0140-6736(91)92907-j. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Oldstone M. B. Protein structure of lymphocytic choriomeningitis virus: evidence for a cell-associated precursor of the virion glycopeptides. Virology. 1979 Nov;99(1):111–120. doi: 10.1016/0042-6822(79)90042-4. [DOI] [PubMed] [Google Scholar]
- Dropulić B., Lin N. H., Martin M. A., Jeang K. T. Functional characterization of a U5 ribozyme: intracellular suppression of human immunodeficiency virus type 1 expression. J Virol. 1992 Mar;66(3):1432–1441. doi: 10.1128/jvi.66.3.1432-1441.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J. B., Scully C. Herpes simplex virus in immunocompromised patients: growing evidence of drug resistance. Oral Surg Oral Med Oral Pathol. 1991 Jul;72(1):47–50. doi: 10.1016/0030-4220(91)90188-i. [DOI] [PubMed] [Google Scholar]
- Epstein L. M., Gall J. G. Self-cleaving transcripts of satellite DNA from the newt. Cell. 1987 Feb 13;48(3):535–543. doi: 10.1016/0092-8674(87)90204-2. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon J. E., Howell R. M., Schwartzer T. A., Gocke D. J., Dubin D. T. In vivo prevalence of azidothymidine (AZT) resistance mutations in an AIDS patient before and after AZT therapy. AIDS Res Hum Retroviruses. 1991 Mar;7(3):265–269. doi: 10.1089/aid.1991.7.265. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Funato T., Yoshida E., Jiao L., Tone T., Kashani-Sabet M., Scanlon K. J. The utility of an anti-fos ribozyme in reversing cisplatin resistance in human carcinomas. Adv Enzyme Regul. 1992;32:195–209. doi: 10.1016/0065-2571(92)90017-t. [DOI] [PubMed] [Google Scholar]
- Gao Q., Gu Z. X., Parniak M. A., Li X. G., Wainberg M. A. In vitro selection of variants of human immunodeficiency virus type 1 resistant to 3'-azido-3'-deoxythymidine and 2',3'-dideoxyinosine. J Virol. 1992 Jan;66(1):12–19. doi: 10.1128/jvi.66.1.12-19.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi G., Gross S. C., Tewari A., Watabe K. Antisense oligodeoxyribonucleotides inhibit the expression of the gene for hepatitis B virus surface antigen. J Gen Virol. 1990 Dec;71(Pt 12):3021–3025. doi: 10.1099/0022-1317-71-12-3021. [DOI] [PubMed] [Google Scholar]
- Gutierrez A. A., Lemoine N. R., Sikora K. Gene therapy for cancer. Lancet. 1992 Mar 21;339(8795):715–721. doi: 10.1016/0140-6736(92)90606-4. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Herschlag D. Implications of ribozyme kinetics for targeting the cleavage of specific RNA molecules in vivo: more isn't always better. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6921–6925. doi: 10.1073/pnas.88.16.6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanov A. V., Vinogradov S. V., Ovcharenko A. V., Krivonos A. V., Melik-Nubarov N. S., Kiselev V. I., Severin E. S. A new class of antivirals: antisense oligonucleotides combined with a hydrophobic substituent effectively inhibit influenza virus reproduction and synthesis of virus-specific proteins in MDCK cells. FEBS Lett. 1990 Jan 1;259(2):327–330. doi: 10.1016/0014-5793(90)80039-l. [DOI] [PubMed] [Google Scholar]
- Klavinskis L. S., Whitton J. L., Joly E., Oldstone M. B. Vaccination and protection from a lethal viral infection: identification, incorporation, and use of a cytotoxic T lymphocyte glycoprotein epitope. Virology. 1990 Oct;178(2):393–400. doi: 10.1016/0042-6822(90)90336-p. [DOI] [PubMed] [Google Scholar]
- Koizumi M., Hayase Y., Iwai S., Kamiya H., Inoue H., Ohtsuka E. Design of RNA enzymes distinguishing a single base mutation in RNA. Nucleic Acids Res. 1989 Sep 12;17(17):7059–7071. doi: 10.1093/nar/17.17.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M., Kamiya H., Ohtsuka E. Ribozymes designed to inhibit transformation of NIH3T3 cells by the activated c-Ha-ras gene. Gene. 1992 Aug 15;117(2):179–184. doi: 10.1016/0378-1119(92)90727-7. [DOI] [PubMed] [Google Scholar]
- Kumar A., Lindberg U. Characterization of messenger ribonucleoprotein and messenger RNA from KB cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):681–685. doi: 10.1073/pnas.69.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter J. M., Agrawal S., Palese P., Zamecnik P. C. Inhibition of influenza virus replication by phosphorothioate oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1990 May;87(9):3430–3434. doi: 10.1073/pnas.87.9.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman P., Ellis M. N., Hackman R. C., Shepp D. H., Meyers J. D. Acyclovir-resistant herpes simplex virus causing pneumonia after marrow transplantation. J Infect Dis. 1990 Jul;162(1):244–248. doi: 10.1093/infdis/162.1.244. [DOI] [PubMed] [Google Scholar]
- Matsukura M., Zon G., Shinozuka K., Robert-Guroff M., Shimada T., Stein C. A., Mitsuya H., Wong-Staal F., Cohen J. S., Broder S. Regulation of viral expression of human immunodeficiency virus in vitro by an antisense phosphorothioate oligodeoxynucleotide against rev (art/trs) in chronically infected cells. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4244–4248. doi: 10.1073/pnas.86.11.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSwiggen J. A., Cech T. R. Stereochemistry of RNA cleavage by the Tetrahymena ribozyme and evidence that the chemical step is not rate-limiting. Science. 1989 May 12;244(4905):679–683. doi: 10.1126/science.2470150. [DOI] [PubMed] [Google Scholar]
- Merigan T. C. Treatment of AIDS with combinations of antiretroviral agents. Am J Med. 1991 Apr 10;90(4A):8S–17S. doi: 10.1016/0002-9343(91)90405-m. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Tishon A., Geckeler R., Lewicki H., Whitton J. L. A common antiviral cytotoxic T-lymphocyte epitope for diverse major histocompatibility complex haplotypes: implications for vaccination. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2752–2755. doi: 10.1073/pnas.89.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Renneisen K., Leserman L., Matthes E., Schröder H. C., Müller W. E. Inhibition of expression of human immunodeficiency virus-1 in vitro by antibody-targeted liposomes containing antisense RNA to the env region. J Biol Chem. 1990 Sep 25;265(27):16337–16342. [PubMed] [Google Scholar]
- Richman D. D. AZT resistance in isolates of HIV. Immunodefic Rev. 1991;2(4):315–318. [PubMed] [Google Scholar]
- Rossi J. J., Sarver N. RNA enzymes (ribozymes) as antiviral therapeutic agents. Trends Biotechnol. 1990 Jul;8(7):179–183. doi: 10.1016/0167-7799(90)90169-x. [DOI] [PubMed] [Google Scholar]
- Ruffner D. E., Stormo G. D., Uhlenbeck O. C. Sequence requirements of the hammerhead RNA self-cleavage reaction. Biochemistry. 1990 Nov 27;29(47):10695–10702. doi: 10.1021/bi00499a018. [DOI] [PubMed] [Google Scholar]
- Salvato M. S., Shimomaye E. M. The completed sequence of lymphocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology. 1989 Nov;173(1):1–10. doi: 10.1016/0042-6822(89)90216-x. [DOI] [PubMed] [Google Scholar]
- Salvato M., Shimomaye E., Oldstone M. B. The primary structure of the lymphocytic choriomeningitis virus L gene encodes a putative RNA polymerase. Virology. 1989 Apr;169(2):377–384. doi: 10.1016/0042-6822(89)90163-3. [DOI] [PubMed] [Google Scholar]
- Salvato M., Shimomaye E., Southern P., Oldstone M. B. Virus-lymphocyte interactions. IV. Molecular characterization of LCMV Armstrong (CTL+) small genomic segment and that of its variant, Clone 13 (CTL-). Virology. 1988 Jun;164(2):517–522. doi: 10.1016/0042-6822(88)90566-1. [DOI] [PubMed] [Google Scholar]
- Sankar S., Cheah K. C., Porter A. G. Antisense oligonucleotide inhibition of encephalomyocarditis virus RNA translation. Eur J Biochem. 1989 Sep 1;184(1):39–45. doi: 10.1111/j.1432-1033.1989.tb14987.x. [DOI] [PubMed] [Google Scholar]
- Sarver N., Cantin E. M., Chang P. S., Zaia J. A., Ladne P. A., Stephens D. A., Rossi J. J. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990 Mar 9;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- Sioud M., Natvig J. B., Førre O. Preformed ribozyme destroys tumour necrosis factor mRNA in human cells. J Mol Biol. 1992 Feb 20;223(4):831–835. doi: 10.1016/0022-2836(92)90244-e. [DOI] [PubMed] [Google Scholar]
- Steinecke P., Herget T., Schreier P. H. Expression of a chimeric ribozyme gene results in endonucleolytic cleavage of target mRNA and a concomitant reduction of gene expression in vivo. EMBO J. 1992 Apr;11(4):1525–1530. doi: 10.1002/j.1460-2075.1992.tb05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Iversen P. L. Inhibition of human immunodeficiency virus type 1-mediated cytopathic effects by poly(L-lysine)-conjugated synthetic antisense oligodeoxyribonucleotides. J Gen Virol. 1989 Oct;70(Pt 10):2673–2682. doi: 10.1099/0022-1317-70-10-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullenger B. A., Lee T. C., Smith C. A., Ungers G. E., Gilboa E. Expression of chimeric tRNA-driven antisense transcripts renders NIH 3T3 cells highly resistant to Moloney murine leukemia virus replication. Mol Cell Biol. 1990 Dec;10(12):6512–6523. doi: 10.1128/mcb.10.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Weerasinghe M., Liem S. E., Asad S., Read S. E., Joshi S. Resistance to human immunodeficiency virus type 1 (HIV-1) infection in human CD4+ lymphocyte-derived cell lines conferred by using retroviral vectors expressing an HIV-1 RNA-specific ribozyme. J Virol. 1991 Oct;65(10):5531–5534. doi: 10.1128/jvi.65.10.5531-5534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J. L., Sheng N., Oldstone M. B., McKee T. A. A "string-of-beads" vaccine, comprising linked minigenes, confers protection from lethal-dose virus challenge. J Virol. 1993 Jan;67(1):348–352. doi: 10.1128/jvi.67.1.348-352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z., Whitton J. L. Ribozymes which cleave arenavirus RNAs: identification of susceptible target sites and inhibition by target site secondary structure. J Virol. 1992 Mar;66(3):1361–1369. doi: 10.1128/jvi.66.3.1361-1369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B., Herschlag D., Cech T. R. Mutations in a nonconserved sequence of the Tetrahymena ribozyme increase activity and specificity. Cell. 1991 Nov 29;67(5):1007–1019. doi: 10.1016/0092-8674(91)90373-7. [DOI] [PubMed] [Google Scholar]