Abstract
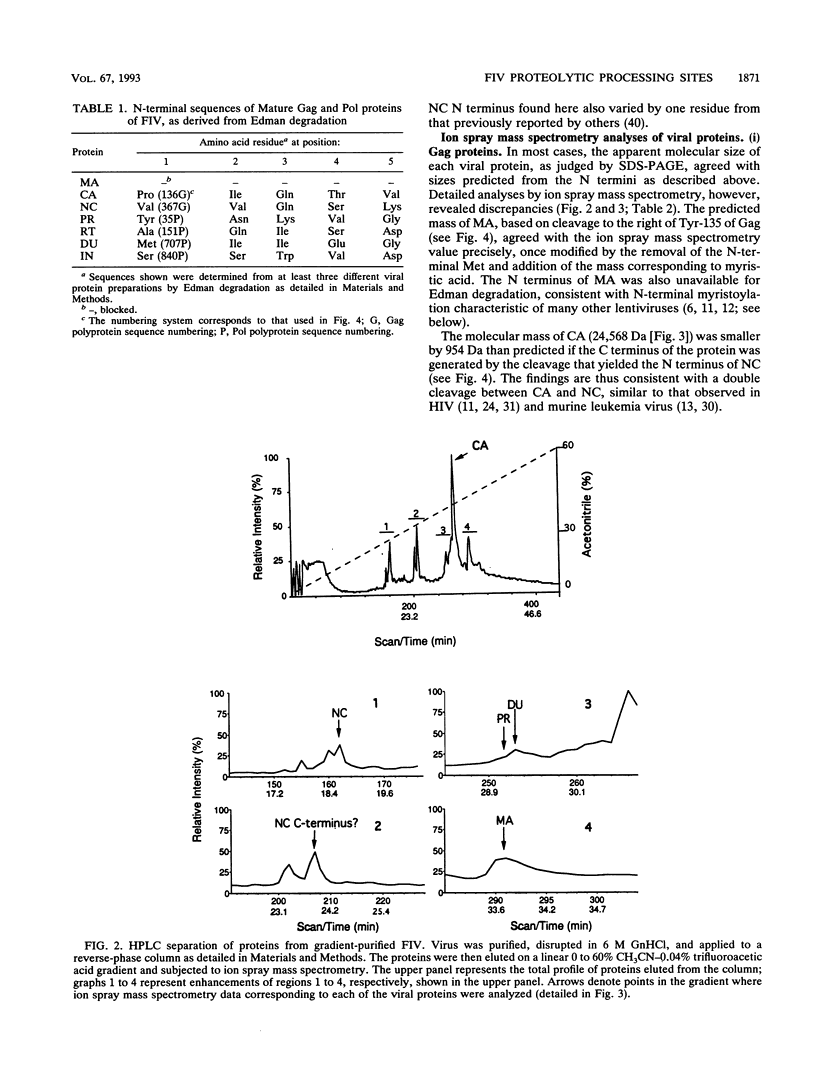
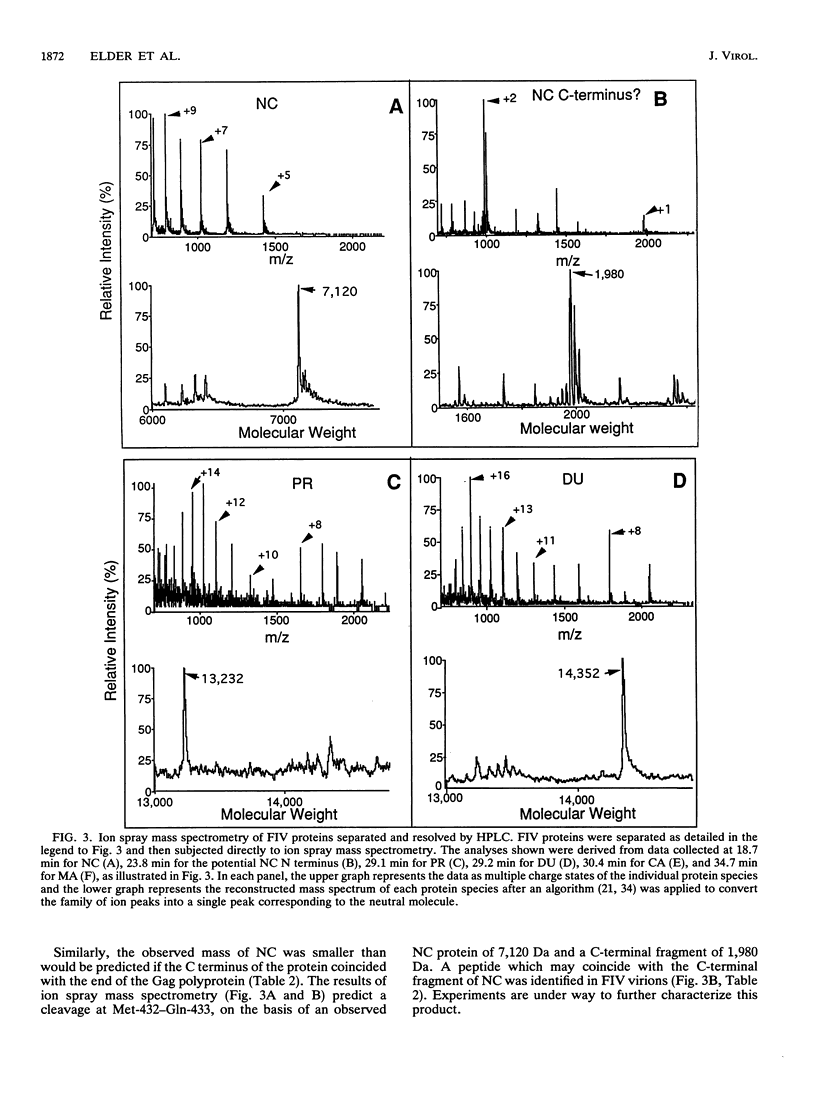
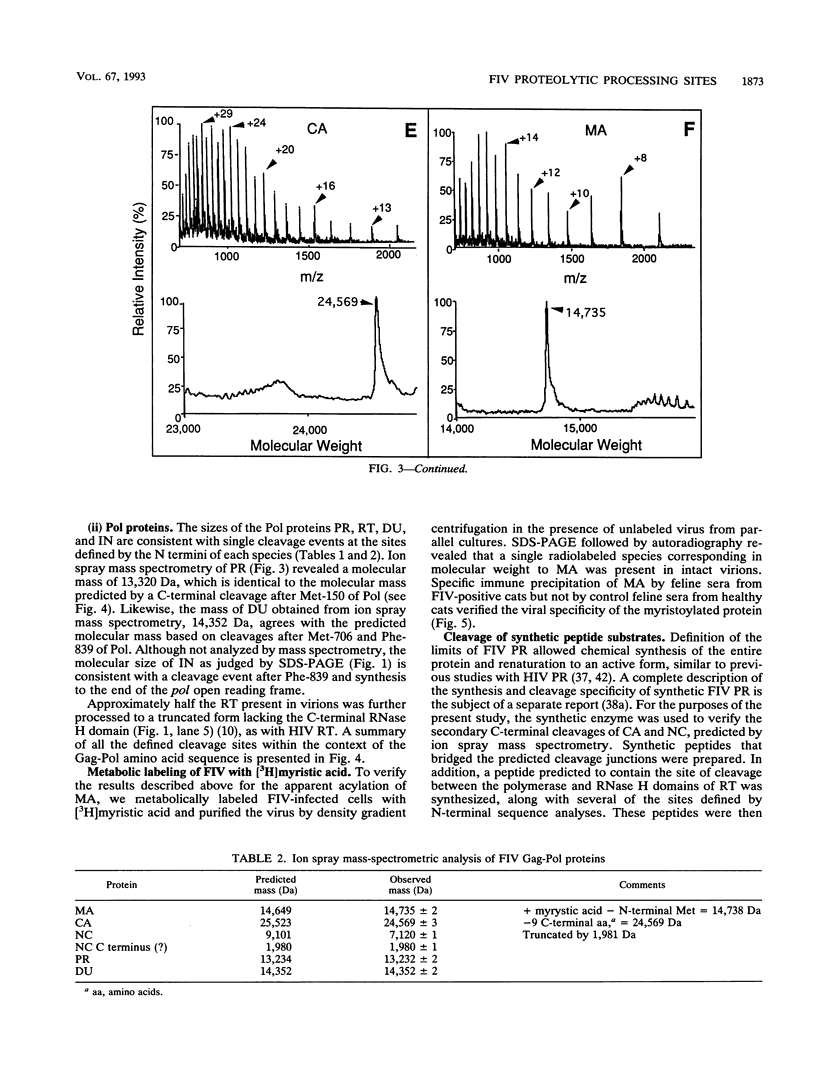
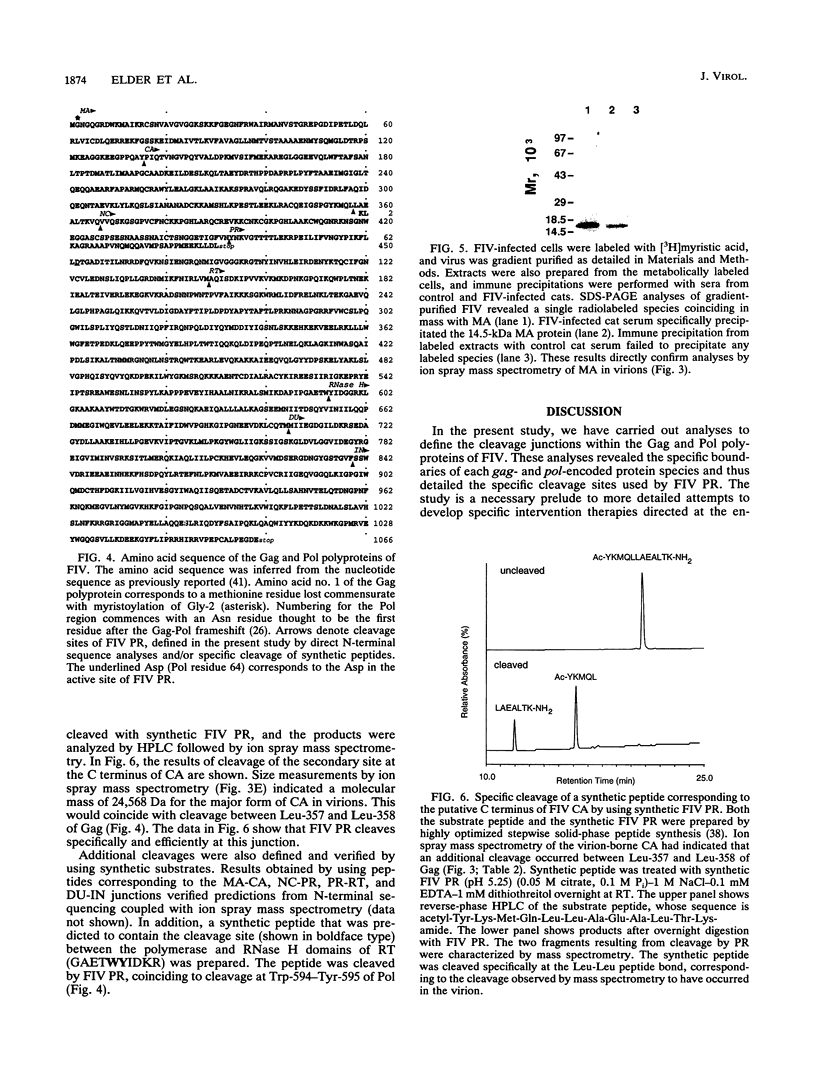
N-terminal amino acid sequencing, ion spray mass spectrometry, and cleavage of synthetic peptide substrates were used to identify the N and C termini of the mature Gag and Pol proteins of feline immunodeficiency virus (FIV). The Gag polyprotein encodes matrix (MA), capsid (CA), and nucleocapsid (NC) proteins. The Gag-Pol polyprotein encodes, in addition to the above proteins, protease (PR), reverse transcriptase (RT), dUTPase (DU), and integrase (IN). Secondary cleavage of RT at Trp-595-Tyr-596 of Pol yields a truncated form lacking the C-terminal RNase H domain. The observed and expected molecular masses of the viral proteins were in agreement, with three exceptions. (i) The molecular mass of MA was 14,735 Da, compared with a predicted mass of 14,649 Da, based on a single cleavage at Tyr-135-Pro-136 of Gag. The observed molecular mass is consistent with myristoylation of MA, which was confirmed by metabolic labeling of FIV MA with [3H]myristic acid. (ii) The N terminus of the NC protein is generated via cleavage at Gln-366-Val-367 of Gag, which predicts a mass of 25,523 for CA and 9,101 for the major form of NC. The observed mass of CA was 24,569, consistent with loss of nine C-terminal amino acids by a second cleavage of CA at Leu-357-Leu-358. Synthetic FIV protease accurately cleaved synthetic peptide substrates containing this site. (iii) The actual mass of NC (7,120 Da) was approximately 2 kDa smaller than the mass predicted by synthesis to the stop codon at the end of Gag (9,101 Da). Experiments are in progress to characterize additional cleavage(s) in NC.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackley C. D., Yamamoto J. K., Levy N., Pedersen N. C., Cooper M. D. Immunologic abnormalities in pathogen-free cats experimentally infected with feline immunodeficiency virus. J Virol. 1990 Nov;64(11):5652–5655. doi: 10.1128/jvi.64.11.5652-5655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold E., Jacobo-Molina A., Nanni R. G., Williams R. L., Lu X., Ding J., Clark A. D., Jr, Zhang A., Ferris A. L., Clark P. Structure of HIV-1 reverse transcriptase/DNA complex at 7 A resolution showing active site locations. Nature. 1992 May 7;357(6373):85–89. doi: 10.1038/357085a0. [DOI] [PubMed] [Google Scholar]
- Baboonian C., Dalgleish A., Bountiff L., Gross J., Oroszlan S., Rickett G., Smith-Burchnell C., Troke P., Merson J. HIV-1 proteinase is required for synthesis of pro-viral DNA. Biochem Biophys Res Commun. 1991 Aug 30;179(1):17–24. doi: 10.1016/0006-291x(91)91327-9. [DOI] [PubMed] [Google Scholar]
- Barlough J. E., Ackley C. D., George J. W., Levy N., Acevedo R., Moore P. F., Rideout B. A., Cooper M. D., Pedersen N. C. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J Acquir Immune Defic Syndr. 1991;4(3):219–227. [PubMed] [Google Scholar]
- Barlow D. J., Edwards M. S., Thornton J. M. Continuous and discontinuous protein antigenic determinants. Nature. 1986 Aug 21;322(6081):747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- Bryant M. L., Ratner L., Duronio R. J., Kishore N. S., Devadas B., Adams S. P., Gordon J. I. Incorporation of 12-methoxydodecanoate into the human immunodeficiency virus 1 gag polyprotein precursor inhibits its proteolytic processing and virus production in a chronically infected human lymphoid cell line. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2055–2059. doi: 10.1073/pnas.88.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford S., Goff S. P. A deletion mutation in the 5' part of the pol gene of Moloney murine leukemia virus blocks proteolytic processing of the gag and pol polyproteins. J Virol. 1985 Mar;53(3):899–907. doi: 10.1128/jvi.53.3.899-907.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillard J. Y., Hoffman T. Enzyme-linked immunosorbent assay for screening monoclonal antibody production using enzyme-labeled second antibody. Methods Enzymol. 1983;92:168–174. doi: 10.1016/0076-6879(83)92016-5. [DOI] [PubMed] [Google Scholar]
- Dow S. W., Poss M. L., Hoover E. A. Feline immunodeficiency virus: a neurotropic lentivirus. J Acquir Immune Defic Syndr. 1990;3(7):658–668. [PubMed] [Google Scholar]
- Elder J. H., Lerner D. L., Hasselkus-Light C. S., Fontenot D. J., Hunter E., Luciw P. A., Montelaro R. C., Phillips T. R. Distinct subsets of retroviruses encode dUTPase. J Virol. 1992 Mar;66(3):1791–1794. doi: 10.1128/jvi.66.3.1791-1794.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Krutzsch H. C., Oroszlan S. Myristyl amino-terminal acylation of murine retrovirus proteins: an unusual post-translational proteins modification. Proc Natl Acad Sci U S A. 1983 Jan;80(2):339–343. doi: 10.1073/pnas.80.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Sowder R., Copeland T. D., Smythers G., Oroszlan S. Quantitative separation of murine leukemia virus proteins by reversed-phase high-pressure liquid chromatography reveals newly described gag and env cleavage products. J Virol. 1984 Nov;52(2):492–500. doi: 10.1128/jvi.52.2.492-500.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T., Washizu T., Toriyabe K., Motoyoshi S., Tomoda I., Pedersen N. C. Feline immunodeficiency virus infection in cats of Japan. J Am Vet Med Assoc. 1989 Jan 15;194(2):221–225. [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Rein A., Shibuya M., Odaka T., Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from "immature" to "mature" core form and for virus infectivity. Virology. 1985 Sep;145(2):280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Kohlstaedt L. A., Wang J., Friedman J. M., Rice P. A., Steitz T. A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992 Jun 26;256(5065):1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu F. T., Zinnecker M., Hamaoka T., Katz D. H. New procedures for preparation and isolation of conjugates of proteins and a synthetic copolymer of D-amino acids and immunochemical characterization of such conjugates. Biochemistry. 1979 Feb 20;18(4):690–693. doi: 10.1021/bi00571a022. [DOI] [PubMed] [Google Scholar]
- Loeb D. D., Hutchison C. A., 3rd, Edgell M. H., Farmerie W. G., Swanstrom R. Mutational analysis of human immunodeficiency virus type 1 protease suggests functional homology with aspartic proteinases. J Virol. 1989 Jan;63(1):111–121. doi: 10.1128/jvi.63.1.111-121.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure M. A., Johnson M. S., Doolittle R. F. Relocation of a protease-like gene segment between two retroviruses. Proc Natl Acad Sci U S A. 1987 May;84(9):2693–2697. doi: 10.1073/pnas.84.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J. Protein sequence comparisons show that the 'pseudoproteases' encoded by poxviruses and certain retroviruses belong to the deoxyuridine triphosphatase family. Nucleic Acids Res. 1990 Jul 25;18(14):4105–4110. doi: 10.1093/nar/18.14.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis R. J., Ahmad N., Lillehoj E. P., Raum M. G., Salazar F. H., Chan H. W., Venkatesan S. The gag gene products of human immunodeficiency virus type 1: alignment within the gag open reading frame, identification of posttranslational modifications, and evidence for alternative gag precursors. J Virol. 1988 Nov;62(11):3993–4002. doi: 10.1128/jvi.62.11.3993-4002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa T., Fukasawa M., Hasegawa A., Maki N., Ikuta K., Takahashi E., Hayami M., Mikami T. Molecular cloning of a novel isolate of feline immunodeficiency virus biologically and genetically different from the original U.S. isolate. J Virol. 1991 Mar;65(3):1572–1577. doi: 10.1128/jvi.65.3.1572-1577.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S., Bishop D. H. Identification and analysis of the gag-pol ribosomal frameshift site of feline immunodeficiency virus. Virology. 1992 Feb;186(2):389–397. doi: 10.1016/0042-6822(92)90004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T. W., Cronn R. C., Remington K. M., Tandberg R. T. Direct comparisons of inhibitor sensitivities of reverse transcriptases from feline and human immunodeficiency viruses. Antimicrob Agents Chemother. 1990 Aug;34(8):1505–1507. doi: 10.1128/aac.34.8.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North T. W., North G. L., Pedersen N. C. Feline immunodeficiency virus, a model for reverse transcriptase-targeted chemotherapy for acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1989 Jun;33(6):915–919. doi: 10.1128/aac.33.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmsted R. A., Hirsch V. M., Purcell R. H., Johnson P. R. Nucleotide sequence analysis of feline immunodeficiency virus: genome organization and relationship to other lentiviruses. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8088–8092. doi: 10.1073/pnas.86.20.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Henderson L. E., Stephenson J. R., Copeland T. D., Long C. W., Ihle J. N., Gilden R. V. Amino- and carboxyl-terminal amino acid sequences of proteins coded by gag gene of murine leukemia virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1404–1408. doi: 10.1073/pnas.75.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Luftig R. B. Retroviral proteinases. Curr Top Microbiol Immunol. 1990;157:153–185. doi: 10.1007/978-3-642-75218-6_6. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Phillips T. R., Talbott R. L., Lamont C., Muir S., Lovelace K., Elder J. H. Comparison of two host cell range variants of feline immunodeficiency virus. J Virol. 1990 Oct;64(10):4605–4613. doi: 10.1128/jvi.64.10.4605-4613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington K. M., Chesebro B., Wehrly K., Pedersen N. C., North T. W. Mutants of feline immunodeficiency virus resistant to 3'-azido-3'-deoxythymidine. J Virol. 1991 Jan;65(1):308–312. doi: 10.1128/jvi.65.1.308-312.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. M., Copeland T. D., Oroszlan S. In situ processing of a retroviral nucleocapsid protein by the viral proteinase. Protein Eng. 1991 Aug;4(6):695–700. doi: 10.1093/protein/4.6.695. [DOI] [PubMed] [Google Scholar]
- Schneider J., Kent S. B. Enzymatic activity of a synthetic 99 residue protein corresponding to the putative HIV-1 protease. Cell. 1988 Jul 29;54(3):363–368. doi: 10.1016/0092-8674(88)90199-7. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S. Fatty acylation of proteins. Annu Rev Cell Biol. 1988;4:611–647. doi: 10.1146/annurev.cb.04.110188.003143. [DOI] [PubMed] [Google Scholar]
- Steinman R., Dombrowski J., O'Connor T., Montelaro R. C., Tonelli Q., Lawrence K., Seymour C., Goodness J., Pedersen N. C., Andersen P. R. Biochemical and immunological characterization of the major structural proteins of feline immunodeficiency virus. J Gen Virol. 1990 Mar;71(Pt 3):701–706. doi: 10.1099/0022-1317-71-3-701. [DOI] [PubMed] [Google Scholar]
- Talbott R. L., Sparger E. E., Lovelace K. M., Fitch W. M., Pedersen N. C., Luciw P. A., Elder J. H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodawer A., Miller M., Jaskólski M., Sathyanarayana B. K., Baldwin E., Weber I. T., Selk L. M., Clawson L., Schneider J., Kent S. B. Conserved folding in retroviral proteases: crystal structure of a synthetic HIV-1 protease. Science. 1989 Aug 11;245(4918):616–621. doi: 10.1126/science.2548279. [DOI] [PubMed] [Google Scholar]
- Yamamoto J. K., Hansen H., Ho E. W., Morishita T. Y., Okuda T., Sawa T. R., Nakamura R. M., Pedersen N. C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989 Jan 15;194(2):213–220. [PubMed] [Google Scholar]
- Yamamoto J. K., Okuda T., Ackley C. D., Louie H., Pembroke E., Zochlinski H., Munn R. J., Gardner M. B. Experimental vaccine protection against feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1991 Nov;7(11):911–922. doi: 10.1089/aid.1991.7.911. [DOI] [PubMed] [Google Scholar]
- Yamamoto J. K., Sparger E., Ho E. W., Andersen P. R., O'Connor T. P., Mandell C. P., Lowenstine L., Munn R., Pedersen N. C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988 Aug;49(8):1246–1258. [PubMed] [Google Scholar]