Abstract
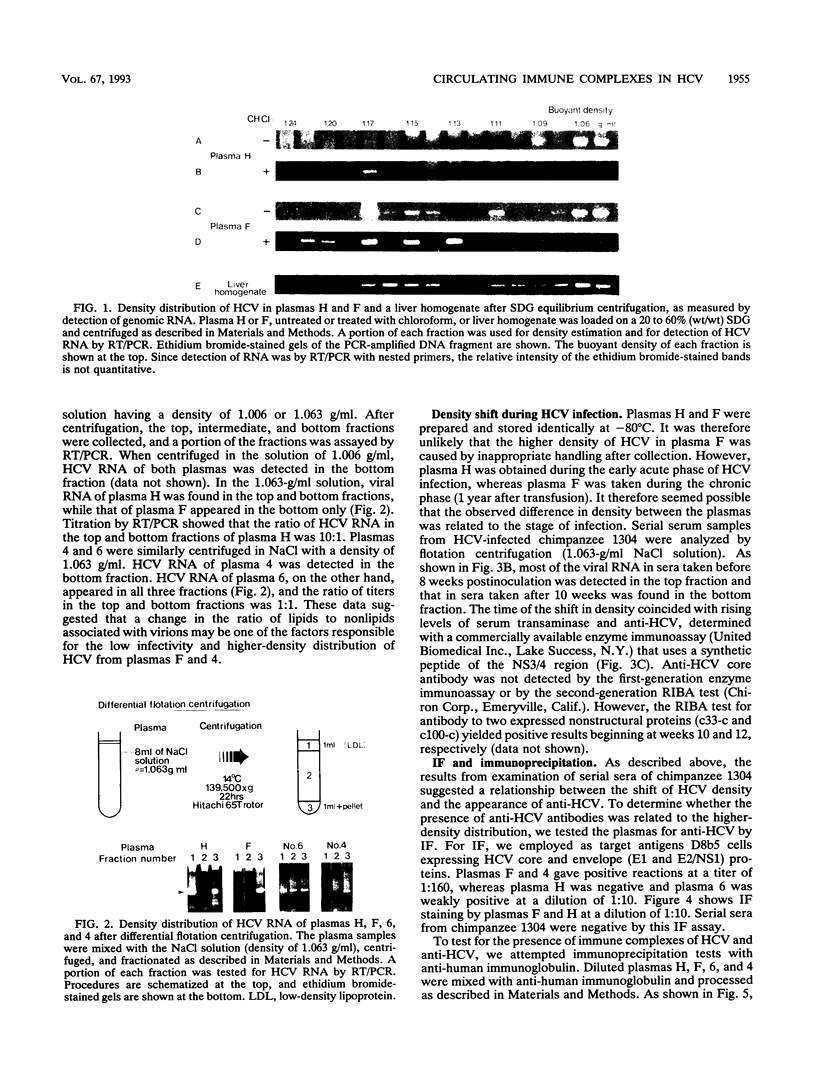
The buoyant density of hepatitis C virus (HCV), with high in vivo infectivity (strain H) or low in vivo infectivity (strain F), was determined by sucrose gradient equilibrium centrifugation. Viral RNA of strain H was detected in fractions with densities of < or = 1.09 g/ml (principally approximately 1.06 g/ml), while that of strain F was found in fractions with densities of approximately 1.06 and approximately 1.17 g/ml. The observed difference was confirmed by differential flotation centrifugation; in NaCl solution with a density of 1.063 g/ml, most of the HCV RNA of strain H was detected in the top fraction, while that of strain F appeared in the bottom. The same relationship between buoyant density and infectivity was observed in flotation centrifugation experiments with other HCV strains. In immunoprecipitation experiments with anti-human immunoglobulin, HCV (as measured by HCV RNA) was precipitated from the samples with low infectivity and high density but not from those with high infectivity and low density. Examination of serial sera from a chimpanzee infected with HCV revealed parallel changes in the buoyant density and immunoprecipitability of HCV-associated RNA during the course of infection. These data suggest that HCV is bound to anti-HCV antibodies as antigen-antibody complexes in chronic hepatitis C.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alter H. J., Purcell R. H., Holland P. V., Popper H. Transmissible agent in non-A, non-B hepatitis. Lancet. 1978 Mar 4;1(8062):459–463. doi: 10.1016/s0140-6736(78)90131-9. [DOI] [PubMed] [Google Scholar]
- Bradley D., McCaustland K., Krawczynski K., Spelbring J., Humphrey C., Cook E. H. Hepatitis C virus: buoyant density of the factor VIII-derived isolate in sucrose. J Med Virol. 1991 Jul;34(3):206–208. doi: 10.1002/jmv.1890340315. [DOI] [PubMed] [Google Scholar]
- Brinton-Darnell M., Plagemann P. G. Structure and chemical-physical characteristics of lactate dehydrogenase-elevating virus and its RNA. J Virol. 1975 Aug;16(2):420–433. doi: 10.1128/jvi.16.2.420-433.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukh J., Purcell R. H., Miller R. H. Sequence analysis of the 5' noncoding region of hepatitis C virus. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4942–4946. doi: 10.1073/pnas.89.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Dienstag J. L., Bhan A. K., Alter H. J., Feinstone S. M., Purcell R. H. Circulating immune complexes in non-A, non-B hepatitis. Possible masking of viral antigen. Lancet. 1979 Jun 16;1(8129):1265–1267. doi: 10.1016/s0140-6736(79)92228-1. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Alter H. J., Dienes H. P., Shimizu Y., Popper H., Blackmore D., Sly D., London W. T., Purcell R. H. Non-A, non-B hepatitis in chimpanzees and marmosets. J Infect Dis. 1981 Dec;144(6):588–598. doi: 10.1093/infdis/144.6.588. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Mihalik K. B., Kamimura T., Alter H. J., London W. T., Purcell R. H. Inactivation of hepatitis B virus and non-A, non-B hepatitis by chloroform. Infect Immun. 1983 Aug;41(2):816–821. doi: 10.1128/iai.41.2.816-821.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L. F., Alling D., Popkin T., Shapiro M., Alter H. J., Purcell R. H. Determining the size of non-A, non-B hepatitis virus by filtration. J Infect Dis. 1987 Oct;156(4):636–640. doi: 10.1093/infdis/156.4.636. [DOI] [PubMed] [Google Scholar]
- Kohara M., Tsukiyama-Kohara K., Maki N., Asano K., Yamaguchi K., Miki K., Tanaka S., Hattori N., Matsuura Y., Saito I. Expression and characterization of glycoprotein gp35 of hepatitis C virus using recombinant vaccinia virus. J Gen Virol. 1992 Sep;73(Pt 9):2313–2318. doi: 10.1099/0022-1317-73-9-2313. [DOI] [PubMed] [Google Scholar]
- Miller R. H., Purcell R. H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H., Okamoto H., Sato K., Tanaka T., Mishiro S. Extraordinarily low density of hepatitis C virus estimated by sucrose density gradient centrifugation and the polymerase chain reaction. J Gen Virol. 1992 Mar;73(Pt 3):715–718. doi: 10.1099/0022-1317-73-3-715. [DOI] [PubMed] [Google Scholar]
- Moritsugu Y., Gold J. W., Wagner J., Dodd R. Y., Purcell R. H. Hepatitis B core antigen. Detection of antibody by radioimmunoprecipitation. J Immunol. 1975 Jun;114(6):1792–1798. [PubMed] [Google Scholar]
- Ogata N., Alter H. J., Miller R. H., Purcell R. H. Nucleotide sequence and mutation rate of the H strain of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3392–3396. doi: 10.1073/pnas.88.8.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Tanaka T., Sugai Y., Akahane Y., Machida A., Mishiro S., Yoshizawa H., Miyakawa Y. Detection of hepatitis C virus RNA by a two-stage polymerase chain reaction with two pairs of primers deduced from the 5'-noncoding region. Jpn J Exp Med. 1990 Aug;60(4):215–222. [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Yotsumoto S., Tanaka T., Yoshizawa H., Tsuda F., Miyakawa Y., Mayumi M. The 5'-terminal sequence of the hepatitis C virus genome. Jpn J Exp Med. 1990 Jun;60(3):167–177. [PubMed] [Google Scholar]
- Shimizu Y. K., Iwamoto A., Hijikata M., Purcell R. H., Yoshikura H. Evidence for in vitro replication of hepatitis C virus genome in a human T-cell line. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5477–5481. doi: 10.1073/pnas.89.12.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y. K., Weiner A. J., Rosenblatt J., Wong D. C., Shapiro M., Popkin T., Houghton M., Alter H. J., Purcell R. H. Early events in hepatitis C virus infection of chimpanzees. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6441–6444. doi: 10.1073/pnas.87.16.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung J. S., Diwan A. R., Falkler W. A., Jr, Yang H. Y., Halstead S. B. Dengue carrier culture and antigen production in human lymphoblastoid lines. Intervirology. 1975;5(3-4):137–149. doi: 10.1159/000149891. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Okamoto H., Kishimoto S., Munekata E., Tachibana K., Akahane Y., Yoshizawa H., Mishiro S. Demonstration of a hepatitis C virus-specific antigen predicted from the putative core gene in the circulation of infected hosts. J Gen Virol. 1992 Mar;73(Pt 3):667–672. doi: 10.1099/0022-1317-73-3-667. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. J., Kuo G., Bradley D. W., Bonino F., Saracco G., Lee C., Rosenblatt J., Choo Q. L., Houghton M. Detection of hepatitis C viral sequences in non-A, non-B hepatitis. Lancet. 1990 Jan 6;335(8680):1–3. doi: 10.1016/0140-6736(90)90134-q. [DOI] [PubMed] [Google Scholar]
- Yoshikura H., Tejima S., Kuchino T., Segawa K., Odaka T. Characterization of N-type and dually permissive cells segregated from mouse fibroblasts whose Fv-1 phenotype could be modified by another independently segregating gene(s). J Virol. 1982 Jan;41(1):145–152. doi: 10.1128/jvi.41.1.145-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]








