Abstract
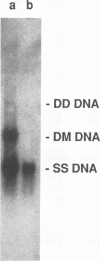
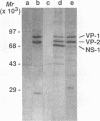
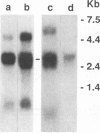
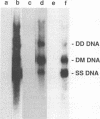
Aleutian mink disease parvovirus (ADV) mRNAs are found in macrophages in lymph nodes and peritoneal exudate cells from ADV-infected mink. Therefore, we developed an in vitro infection system for ADV by using primary cultures of mink macrophages or macrophage cell lines. In peritoneal macrophage cultures from adult mink, virulent ADV-Utah I strain showed nuclear expression of viral antigens with fluorescein isothiocyanate-labeled ADV-infected mink serum, but delineation of specific viral proteins could not be confirmed by immunoblot analysis. Amplification of ADV DNA and production of replicative-form DNA were observed in mink macrophages by Southern blot analysis; however, virus could not be serially propagated. The human macrophage cell line U937 exhibited clear nuclear expression of viral antigens after infection with ADV-Utah I but not with tissue culture-adapted ADV-G. In U937 cells, ADV-Utah I produced mRNA, replicative-form DNA, virion DNA, and structural and nonstructural proteins; however, virus could not be serially passaged nor could [3H]thymidine-labeled virions be observed by density gradient analysis. These findings indicated that ADV-Utah I infection in U937 cells was not fully permissive and that there is another restricted step between gene amplification and/or viral protein expression and production of infectious virions. Treatment with the macrophage activator phorbol 12-myristate 13-acetate after adsorption of virus reduced the frequency of ADV-positive U937 cells but clearly increased that of human macrophage line THP-1 cells. These results suggested that ADV replication may depend on conditions influenced by the differentiation state of macrophages. U937 cells may be useful as an in vitro model system for the analysis of the immune disorder caused by ADV infection of macrophages.
Full text
PDF



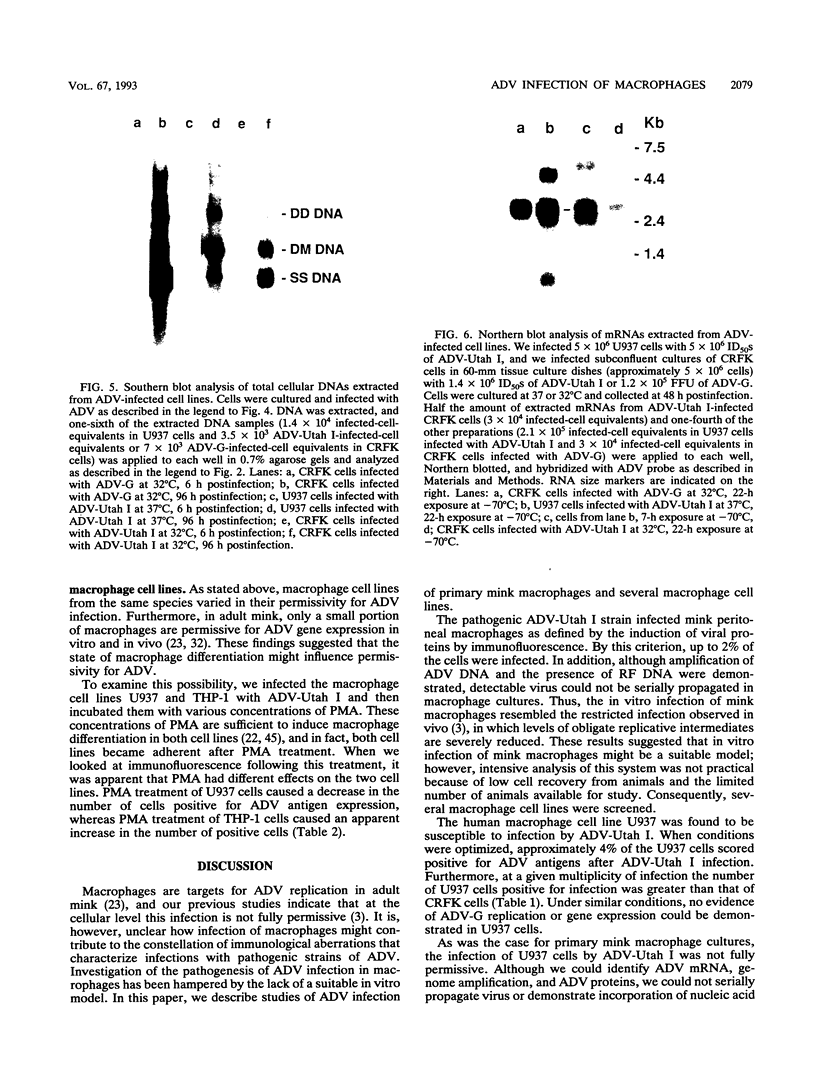
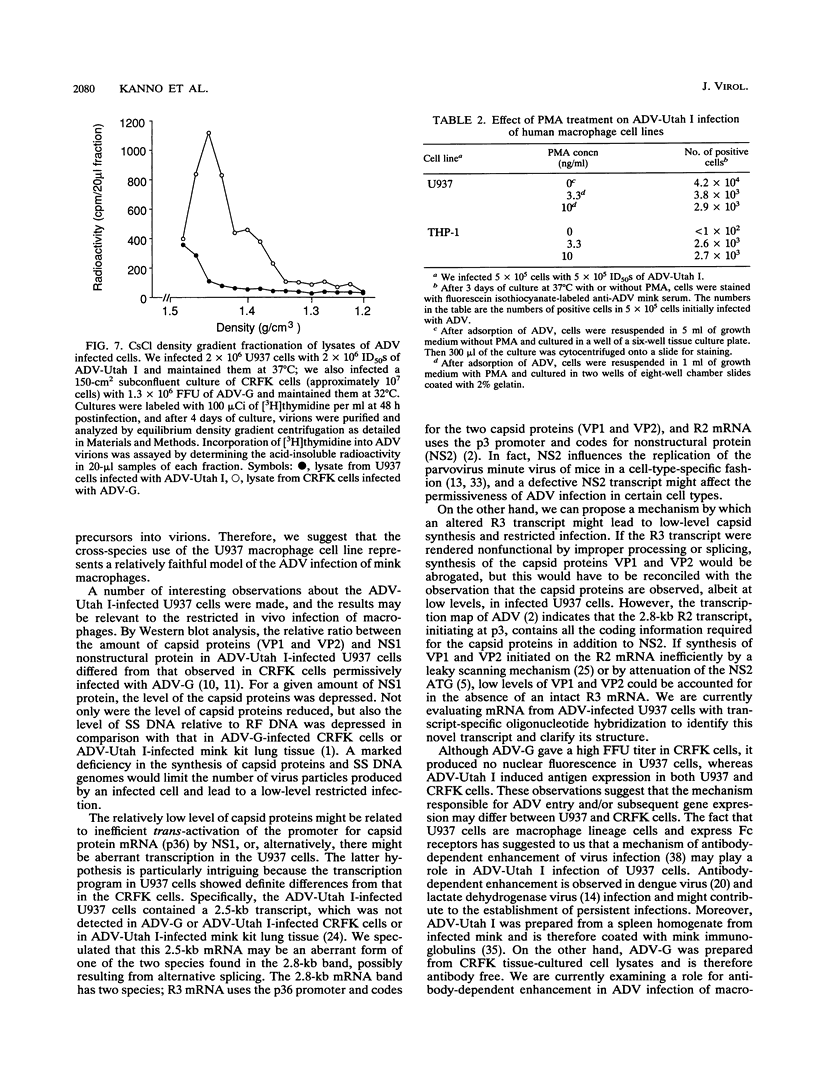


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Bloom M. E., Perryman S. Detailed transcription map of Aleutian mink disease parvovirus. J Virol. 1988 Oct;62(10):3684–3694. doi: 10.1128/jvi.62.10.3684-3694.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E. Studies on the sequential development of acute interstitial pneumonia caused by Aleutian disease virus in mink kits. J Virol. 1987 Jan;61(1):81–86. doi: 10.1128/jvi.61.1.81-86.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J. Evidence of restricted viral replication in adult mink infected with Aleutian disease of mink parvovirus. J Virol. 1988 May;62(5):1495–1507. doi: 10.1128/jvi.62.5.1495-1507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J., Race R. E. In situ molecular hybridization for detection of Aleutian mink disease parvovirus DNA by using strand-specific probes: identification of target cells for viral replication in cell cultures and in mink kits with virus-induced interstitial pneumonia. J Virol. 1987 Aug;61(8):2407–2419. doi: 10.1128/jvi.61.8.2407-2419.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Asher E., Aloni Y. Transcription of minute virus of mice, an autonomous parvovirus, may be regulated by attenuation. J Virol. 1984 Oct;52(1):266–276. doi: 10.1128/jvi.52.1.266-276.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Garon C. F., Mori S., Wei W., Perryman S., Wolfinbarger J. B. Nucleotide sequence of the 5'-terminal palindrome of Aleutian mink disease parvovirus and construction of an infectious molecular clone. J Virol. 1990 Jul;64(7):3551–3556. doi: 10.1128/jvi.64.7.3551-3556.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Perryman S., Lechner D., Wolfinbarger J. B. Nucleotide sequence and genomic organization of Aleutian mink disease parvovirus (ADV): sequence comparisons between a nonpathogenic and a pathogenic strain of ADV. J Virol. 1988 Aug;62(8):2903–2915. doi: 10.1128/jvi.62.8.2903-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Kaaden O. R., Huggans E., Cohn A., Wolfinbarger J. B. Molecular comparisons of in vivo- and in vitro-derived strains of Aleutian disease of mink parvovirus. J Virol. 1988 Jan;62(1):132–138. doi: 10.1128/jvi.62.1.132-138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Mayer L. W., Garon C. F. Characterization of the Aleutian disease virus genome and its intracellular forms. J Virol. 1983 Mar;45(3):977–984. doi: 10.1128/jvi.45.3.977-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Aasted B., Wolfinbarger J. B. Analysis of Aleutian disease virus infection in vitro and in vivo: demonstration of Aleutian disease virus DNA in tissues of infected mink. J Virol. 1985 Sep;55(3):696–703. doi: 10.1128/jvi.55.3.696-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Analysis of Aleutian disease of mink parvovirus infection using strand-specific hybridization probes. Intervirology. 1987;27(2):102–111. doi: 10.1159/000149727. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980 Sep;35(3):836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein D. G., Smith A. L., Johnson E. A., Pintel D. J., Naeger L. K., Tattersall P. The pathogenesis of infection with minute virus of mice depends on expression of the small nonstructural protein NS2 and on the genotype of the allotropic determinants VP1 and VP2. J Virol. 1992 May;66(5):3118–3124. doi: 10.1128/jvi.66.5.3118-3124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafruny W. A., Plagemann P. G. Immune response to lactate dehydrogenase-elevating virus: serologically specific rabbit neutralizing antibody to the virus. Infect Immun. 1982 Sep;37(3):1007–1012. doi: 10.1128/iai.37.3.1007-1012.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Bloom M., Hadlow W., Race R. Purification and ultrastructure of Aleutian disease virus of mink. Nature. 1975 Apr 3;254(5499):456–457. doi: 10.1038/254456a0. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Crandell R. A., Fabricant C. G., Nelson-Rees W. A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK). In Vitro. 1973 Nov-Dec;9(3):176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- Gendelman H. E., Narayan O., Kennedy-Stoskopf S., Kennedy P. G., Ghotbi Z., Clements J. E., Stanley J., Pezeshkpour G. Tropism of sheep lentiviruses for monocytes: susceptibility to infection and virus gene expression increase during maturation of monocytes to macrophages. J Virol. 1986 Apr;58(1):67–74. doi: 10.1128/jvi.58.1.67-74.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B., O'Rourke E. J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977 Jul 1;146(1):201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding M. J., Molitor T. W. Porcine parvovirus: replication in and inhibition of selected cellular functions of swine alveolar macrophages and peripheral blood lymphocytes. Arch Virol. 1988;101(1-2):105–117. doi: 10.1007/BF01314655. [DOI] [PubMed] [Google Scholar]
- Harris P. E., Ralph P., Litcofsky P., Moore M. A. Distinct activities of interferon-gamma, lymphokine and cytokine differentiation-inducing factors acting on the human monoblastic leukemia cell line U937. Cancer Res. 1985 Jan;45(1):9–13. [PubMed] [Google Scholar]
- Kanno H., Wolfinbarger J. B., Bloom M. E. Identification of Aleutian mink disease parvovirus transcripts in macrophages of infected adult mink. J Virol. 1992 Sep;66(9):5305–5312. doi: 10.1128/jvi.66.9.5305-5312.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of protein synthesis in virus-infected animal cells. Adv Virus Res. 1986;31:229–292. doi: 10.1016/S0065-3527(08)60265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathey J. L., Spector S. A. Unrestricted replication of human cytomegalovirus in hydrocortisone-treated macrophages. J Virol. 1991 Nov;65(11):6371–6375. doi: 10.1128/jvi.65.11.6371-6375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrere J. J., Meyer O., Menkes C. J., Beaulieu M. J., Couroucé A. M. Human parvovirus and rheumatoid arthritis. Lancet. 1985 Apr 27;1(8435):982–982. doi: 10.1016/s0140-6736(85)91751-9. [DOI] [PubMed] [Google Scholar]
- Lodmell D. L., Bergman R. K., Bloom M. E., Ewalt L. C., Hadlow W. J., Race R. E. Impaired phagocytosis by the mononuclear phagocytic system in sapphire mink affected with Aleutian disease. Proc Soc Exp Biol Med. 1990 Oct;195(1):75–78. doi: 10.3181/00379727-195-43121. [DOI] [PubMed] [Google Scholar]
- Mauel J., Defendi V. Infection and transformation of mouse peritoneal macrophages by simian virus 40. J Exp Med. 1971 Aug 1;134(2):335–350. doi: 10.1084/jem.134.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. W., Aasted B., Garon C. F., Bloom M. E. Molecular cloning of the Aleutian disease virus genome: expression of Aleutian disease virus antigens by a recombinant plasmid. J Virol. 1983 Dec;48(3):573–579. doi: 10.1128/jvi.48.3.573-579.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Autoinduction of differentiation in WEHI-3B leukemia cells. Int J Cancer. 1982 Dec 15;30(6):773–780. doi: 10.1002/ijc.2910300616. [DOI] [PubMed] [Google Scholar]
- Mori S., Wolfinbarger J. B., Miyazawa M., Bloom M. E. Replication of Aleutian mink disease parvovirus in lymphoid tissues of adult mink: involvement of follicular dendritic cells and macrophages. J Virol. 1991 Feb;65(2):952–956. doi: 10.1128/jvi.65.2.952-956.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeger L. K., Cater J., Pintel D. J. The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J Virol. 1990 Dec;64(12):6166–6175. doi: 10.1128/jvi.64.12.6166-6175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parwaresch M. R., Wacker H. H. Origin and kinetics of resident tissue macrophages. Parabiosis studies with radiolabelled leucocytes. Cell Tissue Kinet. 1984 Jan;17(1):25–39. doi: 10.1111/j.1365-2184.1984.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Cox N. A., Porter H. G., Suffin S. C. Isolation of Aleutian disease virus of mink in cell culture. Intervirology. 1977;8(3):129–144. doi: 10.1159/000148888. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. Aleutian disease of mink. Adv Immunol. 1980;29:261–286. doi: 10.1016/s0065-2776(08)60046-2. [DOI] [PubMed] [Google Scholar]
- Porterfield J. S. Antibody-dependent enhancement of viral infectivity. Adv Virus Res. 1986;31:335–355. doi: 10.1016/s0065-3527(08)60268-7. [DOI] [PubMed] [Google Scholar]
- Ralph P., Prichard J., Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J Immunol. 1975 Feb;114(2 Pt 2):898–905. [PubMed] [Google Scholar]
- Sasaki T., Takahashi Y., Yoshinaga K., Sugamura K., Shiraishi H. An association between human parvovirus B-19 infection and autoantibody production. J Rheumatol. 1989 May;16(5):708–709. [PubMed] [Google Scholar]
- Sawyer R. T., Strausbauch P. H., Volkman A. Resident macrophage proliferation in mice depleted of blood monocytes by strontium-89. Lab Invest. 1982 Feb;46(2):165–170. [PubMed] [Google Scholar]
- Simpson R. W., McGinty L., Simon L., Smith C. A., Godzeski C. W., Boyd R. J. Association of parvoviruses with rheumatoid arthritis of humans. Science. 1984 Mar 30;223(4643):1425–1428. doi: 10.1126/science.6701529. [DOI] [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Kobayashi Y., Goto Y., Okumura H., Nakae S., Konno T., Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982 Apr;42(4):1530–1536. [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Weinshenker B. G., Wilton S., Rice G. P. Phorbol ester-induced differentiation permits productive human cytomegalovirus infection in a monocytic cell line. J Immunol. 1988 Mar 1;140(5):1625–1631. [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A., Hirsch J. G., Humphrey J. H., Spector W. G., Langevoort H. L. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. 1972;46(6):845–852. [PMC free article] [PubMed] [Google Scholar]