Abstract
Xenopus laevis oocytes have been used extensively during the past decade to express and study neurotransmitter receptors of various origins and subunit composition and also to express and study receptors altered by site-specific mutations. Interpretations of the effects of structural differences on receptor mechanisms were, however, hampered by a lack of rapid chemical reaction techniques suitable for use with oocytes. Here we describe flow and photolysis techniques, with 2-ms and 100-μs time resolution, respectively, for studying neurotransmitter receptors in giant (≈20-μm diameter) patches of oocyte membranes, using muscle and neuronal acetylcholine receptors as examples. With these techniques, we find that the muscle receptor in BC3H1 cells and the same receptor expressed in oocytes have comparable kinetic properties. This finding is in contrast to previous studies and raises questions regarding the interpretations of the many studies of receptors expressed in oocytes in which an insufficient time resolution was available. The results obtained indicate that the rapid reaction techniques described here, in conjunction with the oocyte expression system, will be useful in answering many outstanding questions regarding the structure and function of diverse neurotransmitter receptors.
Keywords: flash photolysis, caged neurotransmitter, giant membrane patch
Xenopus laevis oocytes are used extensively to express neurotransmitter receptors from various regions of the nervous system, from different species, and of varying subunit composition and also to express receptors altered by site-specific mutations, nervous system diseases, or exposure to abused drugs (1–5). Neurotransmitters are released at specialized intercellular junctions and bind to receptor proteins, which can form channels through which small inorganic ions cross the plasma membrane. The lifetime and conductance of the open-channel form of the receptors can be measured in oocytes (6) using the single-channel current-recording method (7). Chemical kinetic techniques (8) for measuring elementary steps in the receptor-mediated reactions, which typically occur in the microsecond-to-millisecond time region (reviewed in ref. 9), have been developed for the use with small cells (10–12; reviewed in ref. 9). Such investigations in the much larger oocytes have, however, been hindered by the lack of similar techniques with an appropriate time resolution. The question remained, therefore, whether or not receptors expressed in oocytes have the same properties as the same receptors expressed in their natural cellular environment (for instance, see ref. 5). Questions also remain regarding interpretations of alterations in mechanisms detected by the use of techniques with inadequate time resolution. Two techniques are presently used for measuring kinetic properties of neurotransmitter receptors expressed in oocytes, the two-electrode (13, 14) and the cut-open, Vaseline-gap (15) voltage-clamp methods; they have time resolutions of a few seconds and 40 ms, respectively. In these techniques, the current, a measure of the concentration of open receptor channels, is recorded and analyzed. The time resolution improves when current is recorded from small (diameter of a few micrometers) membrane fragments (in the outside-out patch configuration; ref. 16) containing receptors. However, fewer receptors are present on the small patches, and, consequently, the range of neurotransmitter and inhibitor concentrations that can be used is restricted. At low concentrations of neurotransmitters or high concentrations of inhibitor, one may not obtain an observable current signal. Additionally, reaction intermediates present in low concentrations can be missed.
Investigations of the mammalian muscle acetylcholine receptor in BC3H1 cells demonstrated that a technique with a time resolution in the microsecond-to-millisecond time region is required for evaluating the constants of the elementary reaction steps (10, 12, 17). This time resolution is available for cells with a diameter <30 μm when a cell-flow method (10) and a laser-pulse photolysis technique employing photolabile, biologically inert precursors of neurotransmitters (caged neurotransmitters; refs. 11, 12, 17, and 18) can be used. However, the oocyte diameter is ≈1 mm, and receptors expressed on its surface cannot be equilibrated with a flowing solution of neurotransmitter within a microsecond-to-millisecond time domain (9, 13, 14). In chemical kinetic experiments, the mixing of reactants must be faster than the elementary reaction steps one wants to observe (8). Here we describe the adaptation and application of rapid reaction techniques developed for use with small cells (10–12; reviewed in ref. 9) for use with giant (≈20-μm diameter) patches of oocyte membranes (19, 20). One of the techniques is a flow method and the other involves flash photolysis of caged neurotransmitters. The amount of neurotransmitter released by photolysis is calibrated using the flow method, which also provides an independent verification of some parameters measured by the photolysis method (11). The results obtained are comparable to those obtained previously in extensive chemical kinetic studies with receptor-containing cells (10, 12; reviewed in ref. 9).
To illustrate the approach, we have used the mouse muscle receptor from BC3H1 cells (21–24) and the rat neuronal α7 receptor (25), expressed separately in oocytes. We chose the mouse muscle receptor because it enabled us to compare the results obtained in the oocyte experiments with those from extensive chemical kinetic investigations (10, 12, 17, 18) of the same receptor in BC3H1 cells (26).
MATERIALS AND METHODS
The source of plasmids coding for the mouse muscle and the rat α7 neuronal nicotinic acetylcholine receptors, preparation of the corresponding mRNAs and cDNA, injection of mRNAs for the muscle and cDNA for the α7 receptors into Xenopus oocytes, and measurements using the two-electrode voltage clamp method (13) have been described (27). Currents were recorded from outside-out giant patches of oocyte membrane as described (20). The extracellular solution was the Oocyte Ringer solution-2 (ref. 13; pH 7.4) with the addition of 1-mM CaCl2. The electrode solution used was 135 mM CsF, 5 mM CsCl, 5 mM EGTA, and 10 mM Hepes (pH 7.4). When measurements were made with the α7 neuronal receptor, 50 μM (final concentration) niflumic acid was added to the external buffer (28). All the experiments were performed at −60 mV (pH 7.4; 22°C).
Giant patch electrodes were made from glass capillary tubing (World Precision Instruments, Sarasota, FL). The electrodes were pulled using a Sutter horizontal pipet puller (model p-87; Sutter Instruments, Novato, CA) and fire-polished lightly using a Narishige polisher (model MF-83; Narishige, Tokyo). The average inner diameter of a giant patch electrode was ≈20 μm. The electrode was coated with tocopherol acetate (Sigma) before use. When the electrode was filled with the electrode solution its resistance was ≈150-350 KΩ. Immediately before experiments, the vitelline membrane was dissected away from the oocyte after osmotic shrinking (13) using Oocyte Ringer’s solution supplemented with 1 mM CaCl2 and 300 mM sucrose. A seal was formed between the electrode and the oocyte by gentle negative pressure, which was supplied through a glass syringe connected to a water column. The patch resistance for measurements was in the gigaohm range. The formation of a seal was monitored using the pclamp 6 program (Axon Instruments, Foster City, CA). To avoid recording from endogenous stretch-activated channels (6), the pressure applied to the patch was set to zero using the water column. A cell-flow device (10, 29) was used to equilibrate the receptors with carbamoylcholine or caged carbamoylcholine. The current was detected with an Axopatch-200A amplifier (Axon Instruments), filtered through the built-in bessel RC filter with a cutoff frequency of 2 kHz, and recorded with a sampling frequency of 300–1000 Hz by a Labmaster DMA digitizer (Scientific Solutions, Solon, OH) driven by the pclamp 6 program. The origin program (Microcal Software, Northampton, MA) was used for fitting and plotting the data.
RESULTS AND DISCUSSION
A giant patch (diameter of 20 μm) of oocyte membrane was formed at the tip of an electrode in which the electrode solution contained nigrosine (30). The cell membrane is impermeable to this dye, which was used to enhance the contrast between the background and the glass electrode. The profile of the oocyte membrane patch was photographed through the front camera port of a Zeiss microscope (Axiovert 35) using a ×20 lens (Achrostigmat; Zeiss) (photograph not shown). The profile of the patch at the tip of a glass electrode visualized in this way was flat. Nigrosine was not used in kinetic measurements. To obtain rapid equilibration of neurotransmitter in the flowing solution with the receptors, the direction of solution flow with respect to the receptor-containing membrane is critical—i.e., flowing the neurotransmitter solution parallel to the membrane is the most efficient way to equilibrate the receptors and the neurotransmitter (Fig. 1), because the unstirred layer will be thin and the fluid speed constant (31). When the solution flow is perpendicular to the membrane, equilibration is slower because of the stagnation point that arises in such a flow pattern and hinders access of the flowing solution to the membrane (31). This is illustrated in Fig. 1. A solution of carbamoylcholine (1 mM), a well-characterized analog of acetylcholine, flowed in the parallel (Fig. 1A) or perpendicular (Fig. 1B) direction over muscle receptors in a giant oocyte membrane patch, and the current induced was recorded. With parallel flow the current rise time, an indication of the time it takes for the cell surface receptors to equilibrate with neurotransmitter (carbamoylcholine) in the flowing solution, was on the average 2 ms, which is about one-tenth of the rise time observed with perpendicular flow (20 ms). The influence of the linear flow rate on the rise time was also examined (Fig. 1, legend). The results demonstrate that even with a moderate linear flow rate (see the legend to Fig. 1) a 2-ms rise time can be obtained. This is important because the seal between the membrane and the recording electrode is more stable at low flow rates, and the same membrane patch can be used for as long as several hours.
Figure 1.
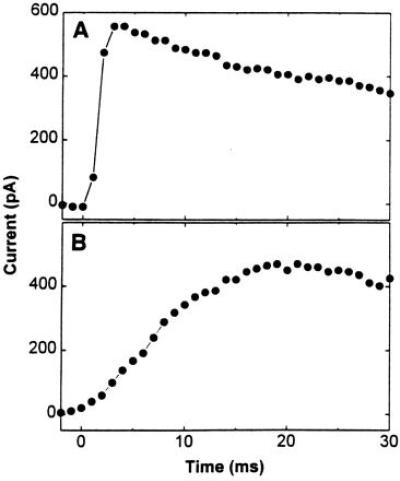
Effects on the rise time of current induced by 1 mM carbamoylcholine solution flowing in different directions with respect to the plane of a giant membrane patch from an oocyte expressing the mouse muscle nicotinic acetylcholine receptor. In A the solution flowed parallel to the giant membrane patch, while in B the solution flowed perpendicular to the same patch. The linear flow rate used in these experiments was 1.2 cm/s. When the parallel flow rate was increased to 3 cm/s, the rise time decreased slightly to 1.8 ms.
The current responses evoked by 1 mM carbamoylcholine and measured using the flow method with giant membrane patches (Fig. 2 A and B, solid lines) and the two-electrode voltage-clamp method with whole oocytes (Fig. 2 A and B, dashed lines) were compared. The measurements in Fig. 2A were made with oocytes expressing the muscle receptor from BC3H1 cells and those in Fig. 2B with oocytes expressing the rat neuronal α7 receptor. To confirm that the carbamoylcholine response was not contaminated with a response activated by the muscarinic receptors intrinsically expressed on Xenopus oocytes (32), we tested the current response to 1 mM carbamoylcholine in the presence and absence of 1 μM atropine, a sufficient amount to inhibit the muscarinic receptors (32), and in each case the current response was similar. The current response obtained in flow experiments with giant oocyte patches (Fig. 2 A and B, solid lines) is entirely different from that obtained in the two-electrode voltage-clamp experiments (Fig. 2 A and B, dashed lines). In Fig. 2A the solid line represents a current with a rise time of 2 ms, while the dashed line represents one of 2 s. In the case of the solid line, the current decay is biphasic with time constants of 21 ms (70%) and 91 ms (30%). In contrast, when a whole oocyte expressing the same type of receptor was used together with the two-electrode voltage-clamp method (Fig. 2A, dashed line), the current decay was slow. Only a single decay phase was detected during a 16-s observation period. The time constant of the current decay is ≈23 s, which is more than two orders of magnitudes larger than the time constant associated with the slow current decay phase observed in the flow experiment with giant membrane patches (Fig. 2A, solid line). Similar observations were made in flow experiments with the neuronal α7 receptor in giant membrane patches (Fig. 2B). The solid line represents a current with a rise time of 2 ms; the desensitization rates were 250 s−1 (75%) and 13 s−1 (25%). In two-electrode voltage-clamp experiments (Fig. 2B, dashed line) done with a whole oocyte containing the same type of receptor, the desensitization rate was 0.3 s−1. In experiments (33) with the α7 receptor expressed in SH-SY5Y cells (diameter of ≈20 μm), with which current rise times of ≈9 ms can be obtained in flow experiments, only a single desensitization rate of over 200 s−1 was detected (estimated from the data in ref. 33).
Figure 2.
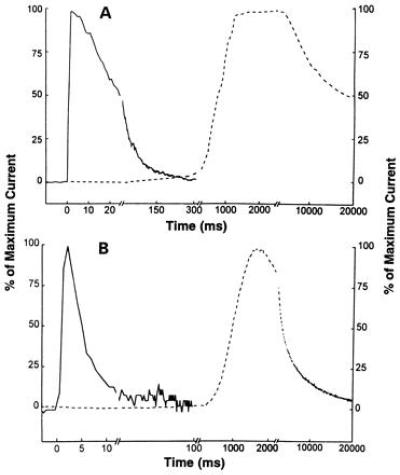
Current responses to 1 mM carbamoylcholine solution flowing over the oocyte membrane patches containing the mouse muscle (A) and rat α7 neuronal (B) acetylcholine receptors at −60 mV (pH 7.4; 22°C). In both A and B, the solid lines represent the current response obtained using giant outside-out oocyte membrane patches with the flow technique described in this paper, and the dotted line represents the response obtained with the whole oocytes two-electrode voltage-clamp technique (13, 14). In A, the abscissa is shown with three different time scales, and the three breaks are from 25 to 27 ms, 308 to 312 ms, and 2200 to 3000 ms. In B, the abscissa is also shown with three different time scales, and the three breaks are from 13 to 15 ms, 108 to 112 ms, and 220 to 2500 ms. The maximum current amplitudes of the responses in A for the muscle receptor are 3.95 nA (solid line) and 17.7 μA (dotted line), and in B, for the α7 neuronal receptor, the amplitudes are 145 pA (solid line) and 94 nA (dotted line).
Using the giant oocyte membrane patches and the flow technique just described, the question of whether the muscle receptor has the same kinetic properties when it is expressed in oocytes as it does in BC3H1 cells was addressed. The results obtained in flow measurements made with giant membrane patches from oocytes expressing the BC3H1 acetylcholine receptor are compared with those obtained in flow, photolysis, and single-channel current measurements made with BC3H1 cells in Fig. 3. There are some differences in the values of the dissociation constants for the site controlling channel opening (K1) and the channel-opening equilibrium constant (Φ; see Fig. 3, legend), but they are not large and may be due to differences in lipid-receptor interactions and/or membrane fluidity (35).
Figure 3.
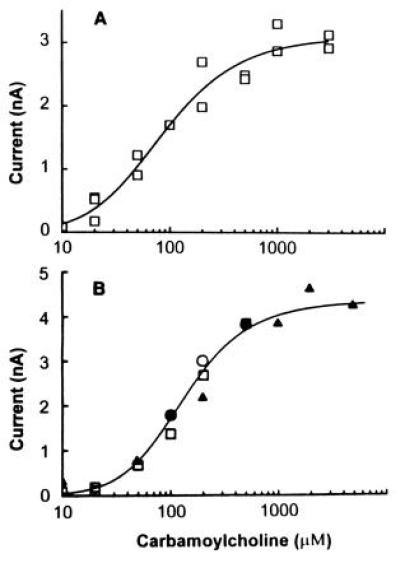
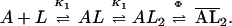 |
 |
 2.
K1 and Φ were calculated to be 48 ± 75
μM and 0.67 ± 1.31, respectively. [The value for
ImRm (10), a measure of the
receptor site density, was calculated to be 3.1 ± 0.2 nA, where
Im is the current per mole of receptor sites and
Rm is the moles of receptor sites in the membrane.]
(B) The dose–response curve determined using the cell-flow
(10) and laser-pulse photolysis (12) methods with the receptor in
BC3H1 cells, under identical conditions as in A,
is shown for comparison. The coordinates for the solid line were
calculated from experimental measurements (see the legend to Fig. 2 in
ref. 12). K1, Φ, and
ImRm were calculated to be 240
μM, 0.17, and 5.1 nA, respectively (12). The triangles represent data
points obtained in cell-flow (10) measurements and the squares data
points obtained by laser-pulse photolysis (12); the circles represent
the fraction of receptors in the open-channel form determined in an
earlier experiment (10) by using the single-channel current-recording
technique (7). [Reproduced with permission from ref. 12 (Copyright
1992, American Chemical Society).]
2.
K1 and Φ were calculated to be 48 ± 75
μM and 0.67 ± 1.31, respectively. [The value for
ImRm (10), a measure of the
receptor site density, was calculated to be 3.1 ± 0.2 nA, where
Im is the current per mole of receptor sites and
Rm is the moles of receptor sites in the membrane.]
(B) The dose–response curve determined using the cell-flow
(10) and laser-pulse photolysis (12) methods with the receptor in
BC3H1 cells, under identical conditions as in A,
is shown for comparison. The coordinates for the solid line were
calculated from experimental measurements (see the legend to Fig. 2 in
ref. 12). K1, Φ, and
ImRm were calculated to be 240
μM, 0.17, and 5.1 nA, respectively (12). The triangles represent data
points obtained in cell-flow (10) measurements and the squares data
points obtained by laser-pulse photolysis (12); the circles represent
the fraction of receptors in the open-channel form determined in an
earlier experiment (10) by using the single-channel current-recording
technique (7). [Reproduced with permission from ref. 12 (Copyright
1992, American Chemical Society).]Another criterion for comparison is receptor desensitization. The rate constant for desensitization determined in flow experiments with giant oocyte membrane patches (Fig. 2A, solid line; 47 s−1) is similar to the value (33 s−1)) reported (36) for an outside-out patch obtained from a BC3H1 cell and exposed to 1-mM carbamoylcholine. The fractions of the current that decayed fast (70%) and slowly (30%) observed in the present studies fall within the range of values obtained previously with BC3H1 cells (10, 12, 17, 18).
A second technique for comparing the properties of the acetylcholine receptor expressed in oocytes and BC3H1 cells was used. The time resolution of the flow technique is too low to determine the rate constants for channel opening and closing. These constants can, however, be evaluated by using a photolysis technique and caged neurotransmitters (11, 12, 17, 18). The technique can also give unique information about drug/receptor interactions that must be measured in the microsecond-to-millisecond time domain (17, 18). The photolysis method using caged carbamoylcholine (11, 12) applied to a giant outside-out oocyte membrane patch is illustrated in Fig. 4. The inactive precursor was photolyzed, using a flash lamp, leading to the release of carbamoylcholine; the resulting current rise, reflecting the opening of receptor channels, was recorded. Standard concentrations of carbamoylcholine flowed from a flow device (10, 29) over the giant patch before and after photolysis to calibrate the concentration of carbamoylcholine liberated and to detect any damage to the receptors. The concentration of free carbamoylcholine generated by photolysis was estimated to be ≈5 μM (Fig. 4, legend). At this low concentration of carbamoylcholine, the observed rate constant for the rise of the current, 345 s−1, reflects the channel-closing rate constant and the lifetime of the channel (12). The value obtained is in good agreement not only with the value of the channel-closing rate constant determined by laser-pulse photolysis with BC3H1 cells (12), but also with the values of the lifetime for the same receptor determined by the single-channel recording technique using BC3H1 cells (37).
Figure 4.
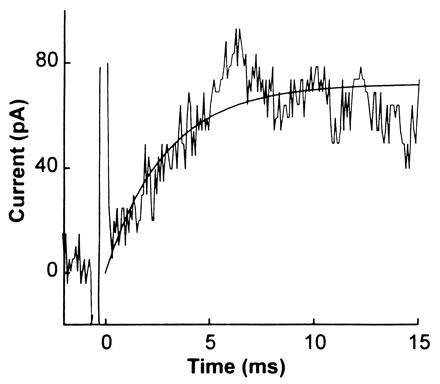
Current response to flash photolysis of 700 μM caged carbamoylcholine equilibrated with an outside-out giant membrane patch from an oocyte expressing the muscle receptor. Caged carbamoylcholine, [N-(α-2-carboxy)-2-nitrobenzyl] carbamoylcholine (11), was dissolved in the extracellular buffer and equilibrated with the muscle receptors. Subsequent photolysis of the caged carbamoylcholine induced a current response with a maximum amplitude of 67 pA. The observed first-order rate constant for the current rise is 345 s−1. The spikes at time zero are instrument artifacts. The light source for photolysis was a flash lamp (Chadwick–Helmuth, El Monte, CA; model 278). The light was coupled into a Zeiss microscope (Axiovert 35) and focused using a ×40 lens (LD Achroplan; Zeiss). The wavelength was in the range 300–390 nm because of the cutoff by the lens and a dichroic mirror used. The pulse energy was ≈700 μJ, as measured by a Joulemeter (Gentec, Palo Alto, CA; model ED-200), for a pulse duration of 300 μs. Labmaster DMA hardware (Scientific Solutions) driven by the pclamp 6 program (Axon Instruments) synchronized triggering of the flash lamp firing and data collection. The current flowing through receptor channels induced to open by the released carbamoylcholine was recorded as described except that the cutoff frequency of the filter was 10–20 kHz, and the sampling frequency was 10–50 kHz. Caged carbamoylcholine (700 μM) was used and the concentration of released carbamoylcholine was estimated to be ≈5 μM using the flow method (10), two known concentrations of carbamoylcholine, 10 and 20 μM (current traces not shown), and the dose–response curve shown in Fig. 3B.
In contrast to the results obtained with rapid reaction techniques are those obtained with the two-electrode voltage-clamp recording technique (Fig. 2, dashed lines); the observed rates of receptor desensitizations were orders of magnitudes lower than those observed with giant oocyte membrane patches (Fig. 2, solid lines) or with small cells with which rapid equilibration between neurotransmitter and cell surface receptors can be obtained (10, 29, 33). We have shown previously that when the equilibration of neurotransmitters in a flowing solution with receptors on the surface of a cell is slow, compared with the rate of receptor desensitization, most of the reaction cannot be observed (reviewed in ref. 9). With the current rise times of seconds in experiments with whole oocytes (Fig. 2, dashed lines), we do not expect to observe the major receptor forms, which all desensitize in the millisecond time region, but are observed in giant oocyte membrane patches or in the cells that represent the natural environment for these receptors. In the case of the muscle nicotinic acetylcholine receptor, it is not known whether the different desensitization rates reflect consecutive desensitizations of the same receptor in different time regions or receptor forms that may differ because of postranslational modification, for instance. In either case, the slowly desensitizing receptor forms can be associated with quite different ligand-binding properties (38). It will, therefore, be of interest to reexamine the interpretations of a large number of previous low time resolution studies of receptor forms expressed in oocytes.
The kinetic approach using giant patches of oocyte membrane described here overcomes the limitations of existing methods and significantly improves the time resolution of the measurements. The 2-ms time resolution provided by the flow method represents a ≈20-fold improvement compared with that obtained in experiments with oocytes using cut-open voltage-clamp recording (15). The time resolution of the flash photolysis technique using giant membrane patches is several orders of magnitudes better than that observed with regular outside-out membrane patches with a diameter of a few micrometers perfused by neurotransmitter solutions (16), and the current response is several orders of magnitude larger. Thus kinetic information that was previously inaccessible with oocytes can now be obtained, including: (i) the rate constants for channel opening and closing (12), (ii) the equilibrium dissociation constant of the neurotransmitter from the receptor (10, 12), and (iii) the effects of inhibitors on the elementary reaction steps (17, 18). One can, therefore, compare these properties of a receptor expressed in oocytes and in its natural environment. In combination with genetic engineering technology, the approach described can be used to investigate how specific amino acid residues and subunits of a receptor influence a variety of functional aspects of receptor activity, including channel activation, ligand binding, and modulation by many different effectors, including abused drugs and therapeutic agents.
Acknowledgments
We thank A. Becker and Dr. J. Rettinger (Max Planck Institute of Biophysics) and Prof. W. Olbricht (Cornell University) for their help in the initial development of the kinetic method using giant membrane patches from oocytes; Dr. A. Costa and Prof. J. Patrick (Baylor College of Medicine) for helpful discussions about the cut-open recording technique; many colleagues for helpful comments on the manuscript; and Susan Coombs for its editing. This research was supported by grants awarded to G.P.H. (NS08527) and to R.E.O. (NS18660) by the National Institutes of Health and by the Council for Tobacco Research, U.S.A., Inc., and to G.P.H. by the National Science Foundation (922061).
References
- 1.Barnard E A, Miledi R, Sumikawa K. Proc R Soc Lond Ser B. 1982;215:241–246. doi: 10.1098/rspb.1982.0040. [DOI] [PubMed] [Google Scholar]
- 2.Samson H H, Harris R A. Trends Pharmacol Sci. 1992;13:206–211. doi: 10.1016/0165-6147(92)90065-e. [DOI] [PubMed] [Google Scholar]
- 3.Villarroel A, Burnashev N, Sakmann B. Biophys J. 1995;68:866–875. doi: 10.1016/S0006-3495(95)80263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonard R J, Labarca C G, Charnet P, Davidson N, Lester H A. Science. 1988;242:1578–1581. doi: 10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- 5.Langosch D, Laube B, Rundström N, Schmieden V, Bormann J, Betz H. EMBO J. 1994;13:4223–4228. doi: 10.1002/j.1460-2075.1994.tb06742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Methfessel C, Witzemann V, Takahashi T, Mishina M, Numa S, Sakmann B. Pfluegers Arch. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- 7.Neher E, Sakmann B. Nature (London) 1976;260:779–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- 8.Gutfreund H. Kinetics for the Life Sciences: Receptors, Transmitters and Catalysts. Cambridge, U.K.: Cambridge Univ. Press; 1995. [Google Scholar]
- 9.Hess G P, Niu L, Wieboldt R. Ann NY Acad Sci. 1995;757:23–39. doi: 10.1111/j.1749-6632.1995.tb17462.x. [DOI] [PubMed] [Google Scholar]
- 10.Udgaonkar J B, Hess G P. Proc Natl Acad Sci USA. 1987;84:8758–8762. doi: 10.1073/pnas.84.24.8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milburn T, Matsubara N, Billington A P, Udgaonkar J B, Walker J W, Webb W W, Marque J, Denk W, McCray J A, Carpenter B K, Hess G P. Biochemistry. 1989;29:49–55. doi: 10.1021/bi00427a008. [DOI] [PubMed] [Google Scholar]
- 12.Matsubara N, Billington A P, Hess G P. Biochemistry. 1992;31:5507–5514. doi: 10.1021/bi00139a012. [DOI] [PubMed] [Google Scholar]
- 13.Bertrand D, Cooper E, Valera S, Rungger D, Ballivet M. Methods Neurosci. 1991;4:174–193. [Google Scholar]
- 14.Stühmer W, Parekh A B. In: Single-Channel Recording. 2nd Ed. Sakmann B, Neher E, editors. New York: Plenum; 1995. pp. 341–356. [Google Scholar]
- 15.Costa A C S, Patrick J W, Dani J A. Biophys J. 1994;67:395–401. doi: 10.1016/S0006-3495(94)80494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pfluegers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 17.Niu L, Hess G P. Biochemistry. 1993;32:3831–3855. doi: 10.1021/bi00066a001. [DOI] [PubMed] [Google Scholar]
- 18.Niu L, Abood L G, Hess G P. Proc Natl Acad Sci USA. 1995;92:12008–12012. doi: 10.1073/pnas.92.26.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilgemann D W. Pfluegers Arch. 1989;415:247–249. doi: 10.1007/BF00370601. [DOI] [PubMed] [Google Scholar]
- 20.Rettinger J, Vasilets L A, Elsner S, Schwartz W. In: The Sodium Pumps. Bamberg E, Schoner W, editors. Darmstadt, Germany: Steikopff; 1994. pp. 553–560. [Google Scholar]
- 21.Merlie J P, Sebbane R, Gardner S, Linstrom J. Proc Natl Acad Sci USA. 1983;80:3845–3849. doi: 10.1073/pnas.80.12.3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaPolla R J, Mayne K M, Davidson N. Proc Natl Acad Sci USA. 1984;81:7970–7974. doi: 10.1073/pnas.81.24.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulter J, Luyten W, Evans K, Mason P, Ballivet M, Goldman D, Stengelin S, Martin G, Heinemann S, Patrick J. J Neurosci. 1985;5:2545–2552. doi: 10.1523/JNEUROSCI.05-09-02545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu L, LaPolla R J, Davidson N. Nucleic Acids Res. 1986;14:3539–3555. doi: 10.1093/nar/14.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seguela P, Wadiche J, Dineley-Miller K, Dani J A, Patrick J W. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert D, Harris D, Devine E E, Heinemann S. J Cell Biol. 1974;61:398–402. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stafford G A, Oswald R E, Weiland G A. Mol Pharmacol. 1994;45:758–762. [PubMed] [Google Scholar]
- 28.Vernino S, Amador M, Luetje C W, Patrick J, Dani J A. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- 29.Krishtal O A, Pidoplichko V I. Neuroscience. 1980;5:2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- 30.Kaltenbach J P, Kaltenbach M H, Lyons W B. Exp Cell Res. 1958;15:112–117. doi: 10.1016/0014-4827(58)90067-3. [DOI] [PubMed] [Google Scholar]
- 31.Tritton D J. Physical Fluid Dynamics. 2nd Ed. Oxford: Clarendon; 1988. [Google Scholar]
- 32.Dascal N, Gillo B, Lass Y. J Physiol (London) 1985;366:299–314. doi: 10.1113/jphysiol.1985.sp015799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puchacz E, Buisson B, Bertrand D, Lukas R J. FEBS Lett. 1994;354:155–159. doi: 10.1016/0014-5793(94)01108-7. [DOI] [PubMed] [Google Scholar]
- 34.Katz B, Thesleff S. J Physiol (London) 1957;138:63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fong T M, McNamee M G. Biochemistry. 1986;25:830–840. doi: 10.1021/bi00352a015. [DOI] [PubMed] [Google Scholar]
- 36.Dilger J P, Liu Y. Pfluegers Arch. 1992;420:479–485. doi: 10.1007/BF00374622. [DOI] [PubMed] [Google Scholar]
- 37.Dilger J P, Brett R S, Poppers D M, Liu Y. Biochim Biophys Acta. 1991;1063:253–258. doi: 10.1016/0005-2736(91)90379-m. [DOI] [PubMed] [Google Scholar]
- 38.Geetha N, Hess G P. Biochemistry. 1992;25:5488–5499. doi: 10.1021/bi00139a010. [DOI] [PubMed] [Google Scholar]


