Abstract
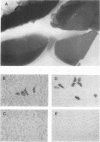
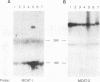
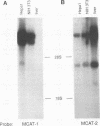
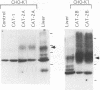
The susceptibility of rodent hepatocytes to infection by mouse type C retroviruses was examined in vivo and in vitro and compared with the expression of two membrane proteins that function as transporters for the cationic amino acids CAT-1 and CAT-2. CAT-1 expression in rodents determines susceptibility to ecotropic retrovirus infection by serving as the virus receptor. Recently, it has been suggested that CAT-2 may be a receptor for amphotropic murine leukemia virus. In the present study, CAT-1 expression was observed in Hepa1, a cell line derived from a murine hepatoma, and in rat hepatocytes propagated on collagen monolayers in vitro but not in intact or regenerating rat liver in vivo. The expression of CAT-1 correlated with susceptibility to infection by an ecotropic retrovirus encoding beta-galactosidase. CAT-2 expression was observed in hepatocytes in vitro and in vivo, consistent with reports of infection of regenerating and cultured hepatocytes by amphotropic retroviruses. However, introduction of murine CAT-2 into nonpermissive Chinese hamster cells was not sufficient to confer susceptibility to amphotropic retrovirus infection, using a protocol that could easily demonstrate CAT-1-dependent infection by an ecotropic virus. Our data establish CAT-1 as a major determinant of ecotropic retrovirus infection in rodent hepatocytes and suggest that CAT-2 is not a receptor for viruses in the amphotropic subgroup.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Kim J. W., Tseng L., Cunningham J. M. Envelope-binding domain in the cationic amino acid transporter determines the host range of ecotropic murine retroviruses. J Virol. 1993 Apr;67(4):2091–2096. doi: 10.1128/jvi.67.4.2091-2096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Armentano D., Thompson A. R., Darlington G., Woo S. L. Expression of human factor IX in rabbit hepatocytes by retrovirus-mediated gene transfer: potential for gene therapy of hemophilia B. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6141–6145. doi: 10.1073/pnas.87.16.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader A., Borel Rinkes I. H., Closs E. I., Ryan C. M., Toner M., Cunningham J. M., Tompkins R. G., Yarmush M. L. A stable long-term hepatocyte culture system for studies of physiologic processes: cytokine stimulation of the acute phase response in rat and human hepatocytes. Biotechnol Prog. 1992 May-Jun;8(3):219–225. doi: 10.1021/bp00015a007. [DOI] [PubMed] [Google Scholar]
- Behr J. P., Demeneix B., Loeffler J. P., Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chowdhury J. R., Grossman M., Gupta S., Chowdhury N. R., Baker J. R., Jr, Wilson J. M. Long-term improvement of hypercholesterolemia after ex vivo gene therapy in LDLR-deficient rabbits. Science. 1991 Dec 20;254(5039):1802–1805. doi: 10.1126/science.1722351. [DOI] [PubMed] [Google Scholar]
- Dunn J. C., Tompkins R. G., Yarmush M. L. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol. 1992 Feb;116(4):1043–1053. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. C., Tompkins R. G., Yarmush M. L. Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog. 1991 May-Jun;7(3):237–245. doi: 10.1021/bp00009a007. [DOI] [PubMed] [Google Scholar]
- Dunn J. C., Yarmush M. L., Koebe H. G., Tompkins R. G. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989 Feb;3(2):174–177. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- Elsdale T., Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972 Sep;54(3):626–637. doi: 10.1083/jcb.54.3.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enat R., Jefferson D. M., Ruiz-Opazo N., Gatmaitan Z., Leinwand L. A., Reid L. M. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. V., Jones C., Miller A. D. Localization of the amphotropic murine leukemia virus receptor gene to the pericentromeric region of human chromosome 8. J Virol. 1991 Nov;65(11):6316–6319. doi: 10.1128/jvi.65.11.6316-6319.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzoglou M., Park E., Wynshaw-Boris A., Kaung H. L., Hanson R. W. Hormonal regulation of chimeric genes containing the phosphoenolpyruvate carboxykinase promoter regulatory region in hepatoma cells infected by murine retroviruses. J Biol Chem. 1988 Nov 25;263(33):17798–17808. [PubMed] [Google Scholar]
- Jaenisch R., Hoffmann E. Transcription of endogenous C-type viruses in resting and proliferating tissues of BALB/Mo mice. Virology. 1979 Oct 30;98(2):289–297. doi: 10.1016/0042-6822(79)90552-x. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Retroviruses and embryogenesis: microinjection of Moloney leukemia virus into midgestation mouse embryos. Cell. 1980 Jan;19(1):181–188. doi: 10.1016/0092-8674(80)90399-2. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Kreamer B. L., Staecker J. L., Sawada N., Sattler G. L., Hsia M. T., Pitot H. C. Use of a low-speed, iso-density percoll centrifugation method to increase the viability of isolated rat hepatocyte preparations. In Vitro Cell Dev Biol. 1986 Apr;22(4):201–211. doi: 10.1007/BF02623304. [DOI] [PubMed] [Google Scholar]
- Land H., Chen A. C., Morgenstern J. P., Parada L. F., Weinberg R. A. Behavior of myc and ras oncogenes in transformation of rat embryo fibroblasts. Mol Cell Biol. 1986 Jun;6(6):1917–1925. doi: 10.1128/mcb.6.6.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledley F. D., Darlington G. J., Hahn T., Woo S. L. Retroviral gene transfer into primary hepatocytes: implications for genetic therapy of liver-specific functions. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5335–5339. doi: 10.1073/pnas.84.15.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C. L., Finley K., Kakuda D., Kozak C. A., Wilkinson M. F. Activated T cells express a novel gene on chromosome 8 that is closely related to the murine ecotropic retroviral receptor. Mol Cell Biol. 1990 Jul;10(7):3663–3674. doi: 10.1128/mcb.10.7.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanohara A., Sharkey M. F., Witztum J. L., Steinberg D., Friedmann T. Efficient expression of retroviral vector-transduced human low density lipoprotein (LDL) receptor in LDL receptor-deficient rabbit fibroblasts in vitro. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6538–6542. doi: 10.1073/pnas.85.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozga J., Moscioni A. D., Neuzil D., Demetriou A. A. A model for directed foreign gene delivery to rat liver cells in vivo. J Surg Res. 1992 Mar;52(3):209–213. doi: 10.1016/0022-4804(92)90075-b. [DOI] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Christensen H. N. Cationic amino acid transport into cultured animal cells. II. Transport system barely perceptible in ordinary hepatocytes, but active in hepatoma cell lines. J Biol Chem. 1982 Apr 25;257(8):4450–4457. [PubMed] [Google Scholar]
- White M. F. The transport of cationic amino acids across the plasma membrane of mammalian cells. Biochim Biophys Acta. 1985 Dec 9;822(3-4):355–374. doi: 10.1016/0304-4157(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Jefferson D. M., Chowdhury J. R., Novikoff P. M., Johnston D. E., Mulligan R. C. Retrovirus-mediated transduction of adult hepatocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3014–3018. doi: 10.1073/pnas.85.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Yee J. K., Skelly H. F., Moores J. C., Respess J. G., Friedmann T., Leffert H. Expression of retrovirally transduced genes in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1987 May;84(10):3344–3348. doi: 10.1073/pnas.84.10.3344. [DOI] [PMC free article] [PubMed] [Google Scholar]