Abstract
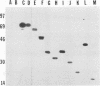
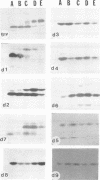
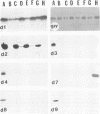
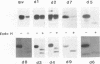
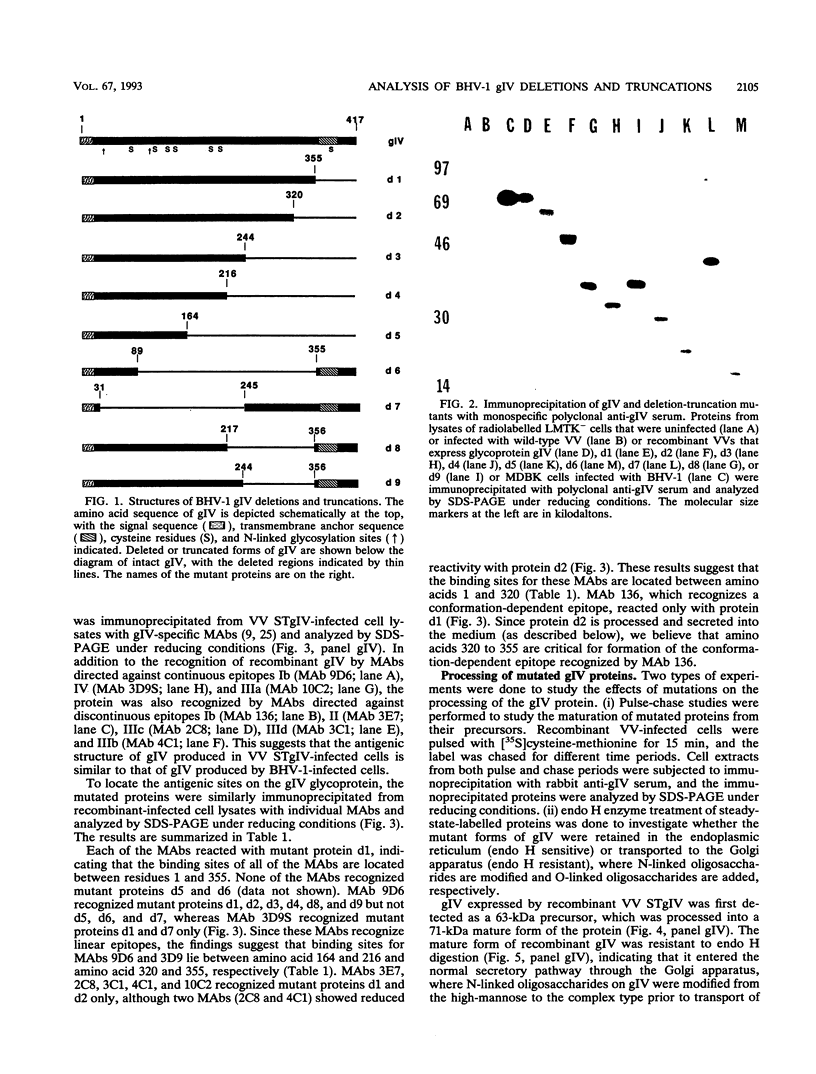
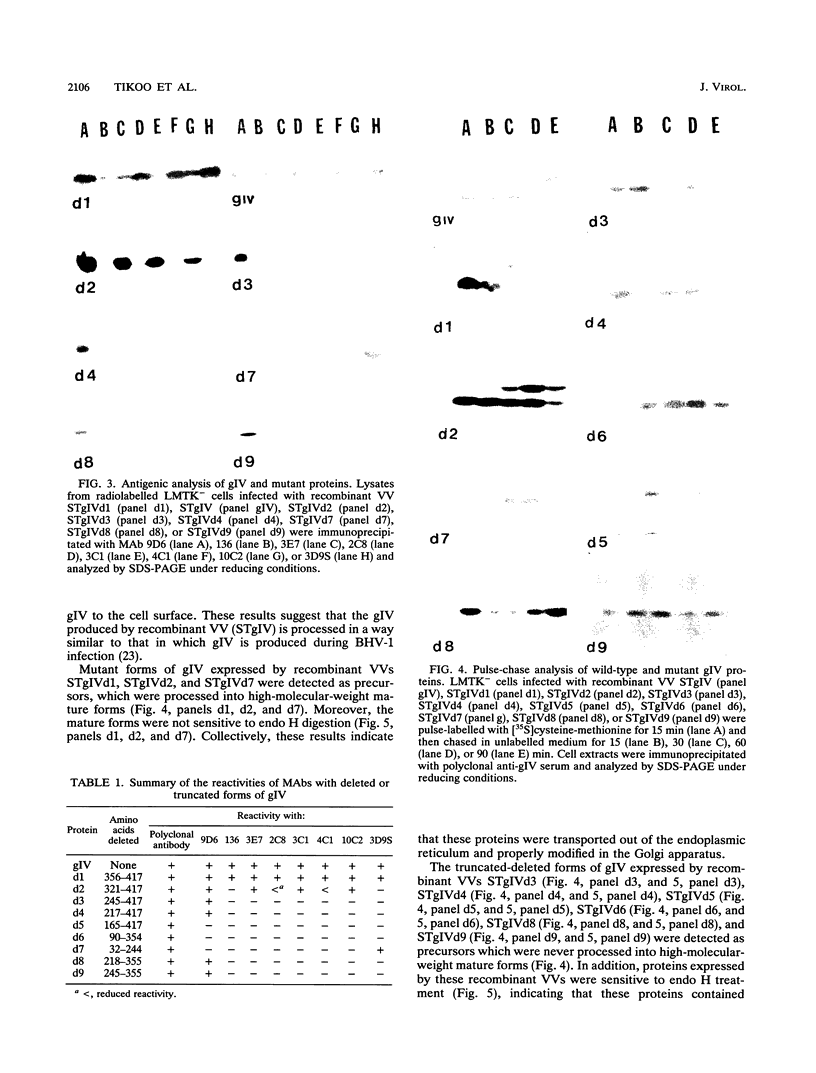
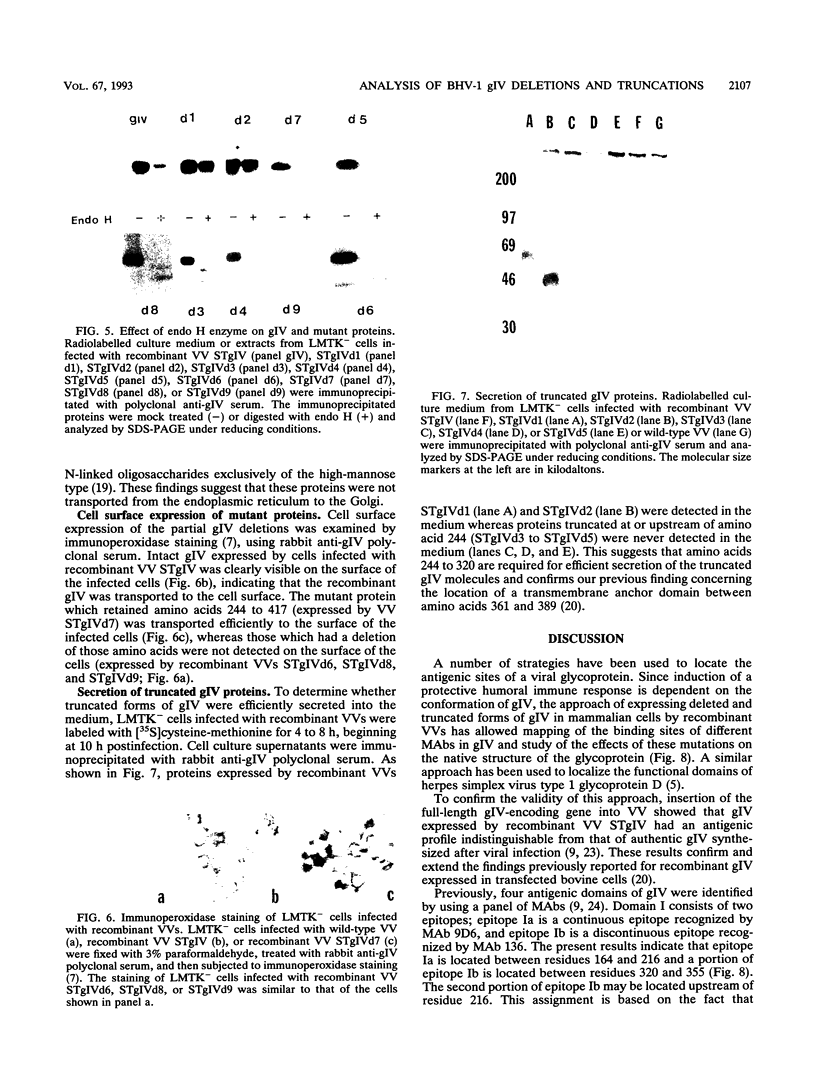
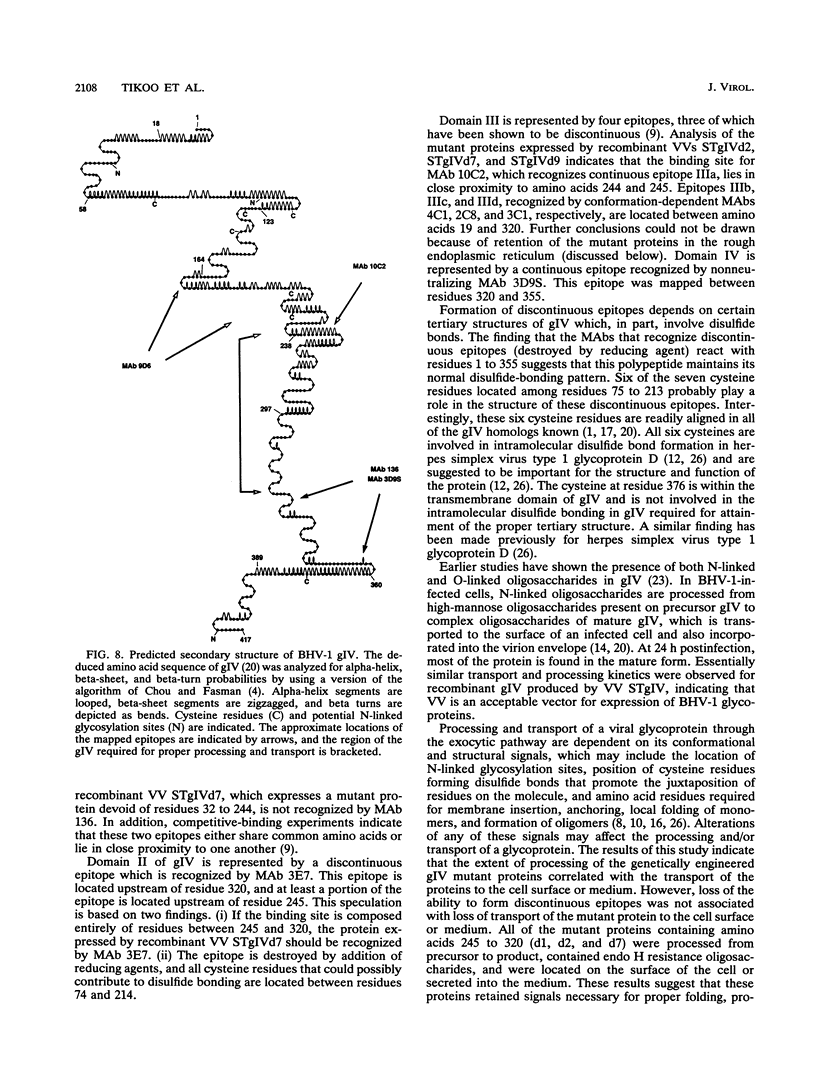
Glycoprotein gIV is an envelope component of bovine herpesvirus type 1 and appears to be involved in attachment, penetration, and cell fusion. Four antigenic domains which include both continuous and discontinuous epitopes have been previously defined by competition binding assays using gIV-specific monoclonal antibodies (MAbs). Here we describe the construction of C-terminal truncations and internal deletions in the gIV-encoding gene and analyses of the effects of these mutations on the synthesis, processing, transport, and antigenicity of glycoprotein gIV as expressed by recombinant vaccinia viruses. Wild-type gIV expressed by recombinant vaccinia virus STgIV was indistinguishable from authentic gIV produced in bovine herpesvirus 1-infected cells with respect to molecular weight, processing, transport, and antigenicity. Analysis of the mutant proteins showed that the binding sites for MAbs 9D6 and 3D9S, which recognize linear epitopes, lie between amino acids 164 and 216 and amino acids 320 and 355, respectively. Discontinuous epitopes recognized by MAbs 3E7, 4C1, 2C8, and 3C1 were located between amino acids 19 and 320, whereas amino acids 320 to 355 were critical for binding of MAb 136. All mutant proteins containing amino acids 245 to 320 were processed, possess endo-beta-N-acetylglucosaminidase H-resistant oligosaccharides, and were transported to the cell surface or secreted into the medium. In contrast, mutant proteins missing amino acids 245 to 320 were retained in the rough endoplasmic reticulum. These findings suggest that residues 245 to 320 are important for proper processing and transport of gIV to the cell surface.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audonnet J. C., Winslow J., Allen G., Paoletti E. Equine herpesvirus type 1 unique short fragment encodes glycoproteins with homology to herpes simplex virus type 1 gD, gI and gE. J Gen Virol. 1990 Dec;71(Pt 12):2969–2978. doi: 10.1099/0022-1317-71-12-2969. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., L'Italien J., van Drunen Littel-van den Hurk S., Zamb T., Lawman J. P., Hughes G., Gifford G. A. Protection of cattle from bovine herpesvirus type I (BHV-1) infection by immunization with individual viral glycoproteins. Virology. 1987 Jul;159(1):57–66. doi: 10.1016/0042-6822(87)90347-3. [DOI] [PubMed] [Google Scholar]
- Barany F. Two-codon insertion mutagenesis of plasmid genes by using single-stranded hexameric oligonucleotides. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4202–4206. doi: 10.1073/pnas.82.12.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Wilcox W. C., Sodora D. L., Long D., Levin J. Z., Eisenberg R. J. Expression of herpes simplex virus type 1 glycoprotein D deletion mutants in mammalian cells. J Virol. 1988 Jun;62(6):1932–1940. doi: 10.1128/jvi.62.6.1932-1940.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehler F., Herrmann J. M., Saalmüller A., Mettenleiter T. C., Keil G. M. Glycoprotein IV of bovine herpesvirus 1-expressing cell line complements and rescues a conditionally lethal viral mutant. J Virol. 1992 Feb;66(2):831–839. doi: 10.1128/jvi.66.2.831-839.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick D. R., Zamb T., Parker M. D., van Drunen Littel-van den Hurk S., Babiuk L. A., Lawman M. J. Expression of bovine herpesvirus 1 glycoproteins gI and gIII in transfected murine cells. J Virol. 1988 Nov;62(11):4239–4248. doi: 10.1128/jvi.62.11.4239-4248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Machamer C. E., Rose J. K. Glycosylation allows cell-surface transport of an anchored secretory protein. Cell. 1985 Sep;42(2):489–496. doi: 10.1016/0092-8674(85)90106-0. [DOI] [PubMed] [Google Scholar]
- Hughes G., Babiuk L. A., van Drunen Littel-van den Hurk S. Functional and topographical analyses of epitopes on bovine herpesvirus type 1 glycoprotein IV. Arch Virol. 1988;103(1-2):47–60. doi: 10.1007/BF01319808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. P., Babiuk L. A., van Drunen Littel-van den Hurk S., Fitzpatrick D. R., Zamb T. J. Bovine herpesvirus 1 attachment to permissive cells is mediated by its major glycoproteins gI, gIII, and gIV. J Virol. 1991 Mar;65(3):1124–1132. doi: 10.1128/jvi.65.3.1124-1132.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D., Cohen G. H., Muggeridge M. I., Eisenberg R. J. Cysteine mutants of herpes simplex virus type 1 glycoprotein D exhibit temperature-sensitive properties in structure and function. J Virol. 1990 Nov;64(11):5542–5552. doi: 10.1128/jvi.64.11.5542-5552.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. L., Rodriguez L. L., Letchworth G. J., 3rd Characterization of envelope proteins of infectious bovine rhinotracheitis virus (bovine herpesvirus 1) by biochemical and immunological methods. J Virol. 1986 Mar;57(3):745–753. doi: 10.1128/jvi.57.3.745-753.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Ross L. J., Binns M. M. Properties and evolutionary relationships of the Marek's disease virus homologues of protein kinase, glycoprotein D and glycoprotein I of herpes simplex virus. J Gen Virol. 1991 Apr;72(Pt 4):939–947. doi: 10.1099/0022-1317-72-4-939. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Babiuk L. A. Host responses to infectious bovine rhinotracheitis virus. III. Isolation and immunologic activities of bovine T lymphocytes. J Immunol. 1974 Nov;113(5):1391–1398. [PubMed] [Google Scholar]
- Tikoo S. K., Fitzpatrick D. R., Babiuk L. A., Zamb T. J. Molecular cloning, sequencing, and expression of functional bovine herpesvirus 1 glycoprotein gIV in transfected bovine cells. J Virol. 1990 Oct;64(10):5132–5142. doi: 10.1128/jvi.64.10.5132-5142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikoo S. K., Parker M. D., van den Hurk J. V., Kowalski J., Zamb T. J., Babiuk L. A. Role of N-linked glycans in antigenicity, processing, and cell surface expression of bovine herpesvirus 1 glycoprotein gIV. J Virol. 1993 Feb;67(2):726–733. doi: 10.1128/jvi.67.2.726-733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox W. C., Long D., Sodora D. L., Eisenberg R. J., Cohen G. H. The contribution of cysteine residues to antigenicity and extent of processing of herpes simplex virus type 1 glycoprotein D. J Virol. 1988 Jun;62(6):1941–1947. doi: 10.1128/jvi.62.6.1941-1947.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Babiuk L. A. Synthesis and processing of bovine herpesvirus 1 glycoproteins. J Virol. 1986 Aug;59(2):401–410. doi: 10.1128/jvi.59.2.401-410.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., Hughes G., Babiuk L. A. The role of carbohydrate in the antigenic and immunogenic structure of bovine herpesvirus type 1 glycoproteins gI and gIV. J Gen Virol. 1990 Sep;71(Pt 9):2053–2063. doi: 10.1099/0022-1317-71-9-2053. [DOI] [PubMed] [Google Scholar]
- van Drunen Littel-van den Hurk S., van den Hurk J. V., Gilchrist J. E., Misra V., Babiuk L. A. Interactions of monoclonal antibodies and bovine herpesvirus type 1 (BHV-1) glycoproteins: characterization of their biochemical and immunological properties. Virology. 1984 Jun;135(2):466–479. doi: 10.1016/0042-6822(84)90201-0. [DOI] [PubMed] [Google Scholar]