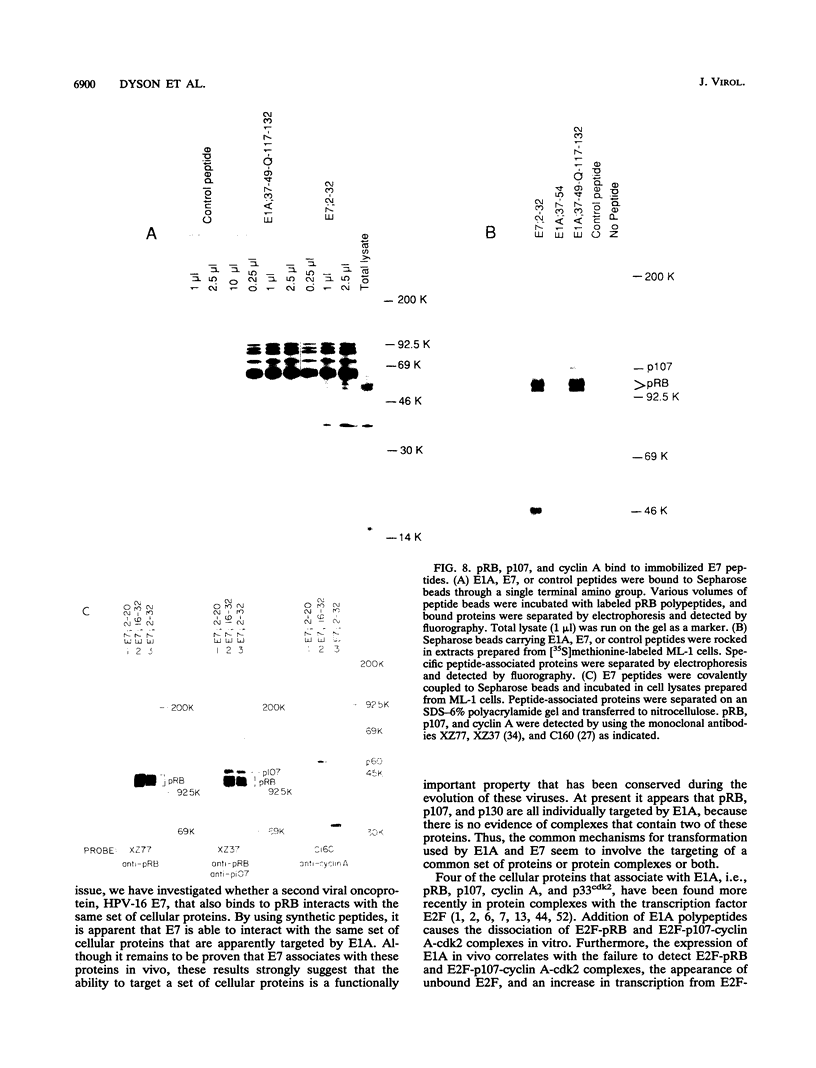
Abstract
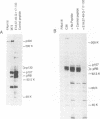
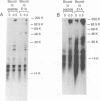
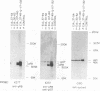
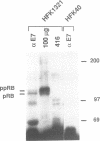
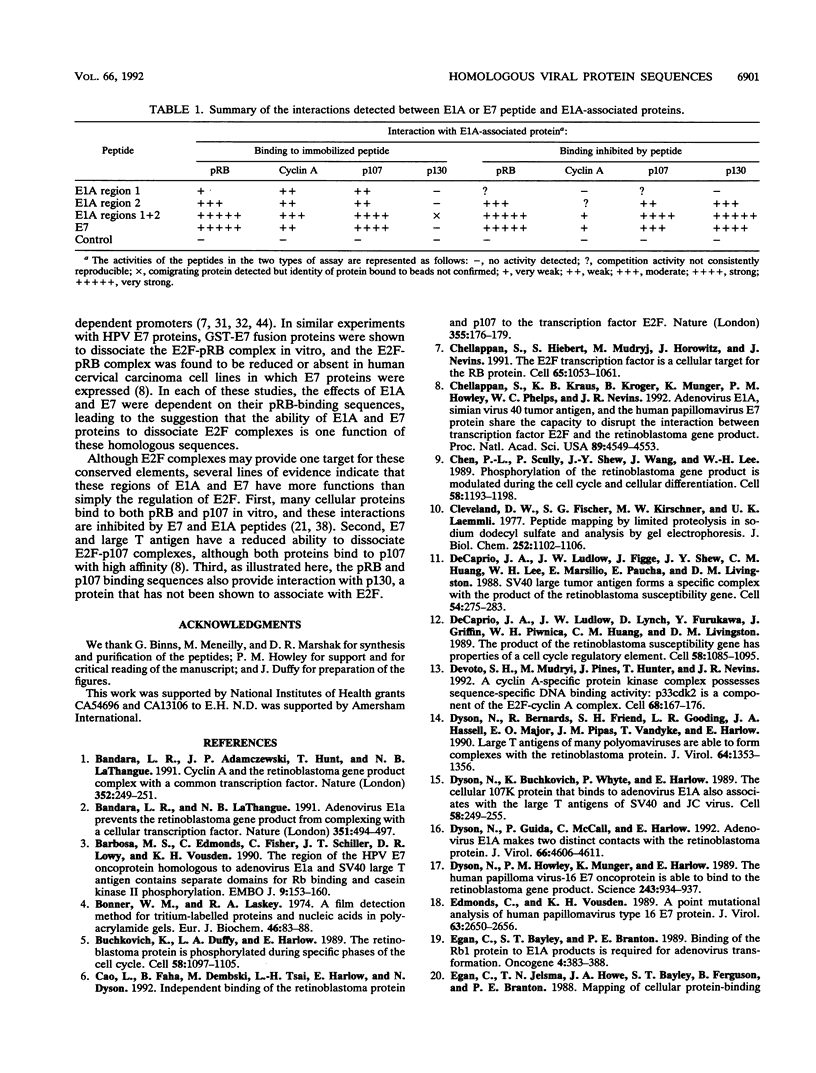
Studies of adenovirus E1A oncoprotein mutants suggest that the association of E1A with the retinoblastoma protein (pRB) is necessary for E1A-mediated transformation. Mutational analysis of E1A indicates that two regions of pRB are required for E1A to form stable complexes with the retinoblastoma protein. In addition to pRB binding, these regions are necessary for E1A association with several other cellular proteins, including p130, p107, cyclin A, and p33cdk2. Here we show that short synthetic peptides containing the pRB-binding sequences of E1A are sufficient for interaction with p107, cyclin A, and p130. The E7 protein of human papillomavirus type 16 contains an element that binds to pRB and appears to be functionally homologous to the E1A sequences. Peptides containing this region of the E7 protein were able to interact with p107, cyclin A, and p130 in addition to pRB. These findings suggest that the common mechanism of transformation used by these viral oncogenes involves their association with a set of polypeptides.
Full text
PDF

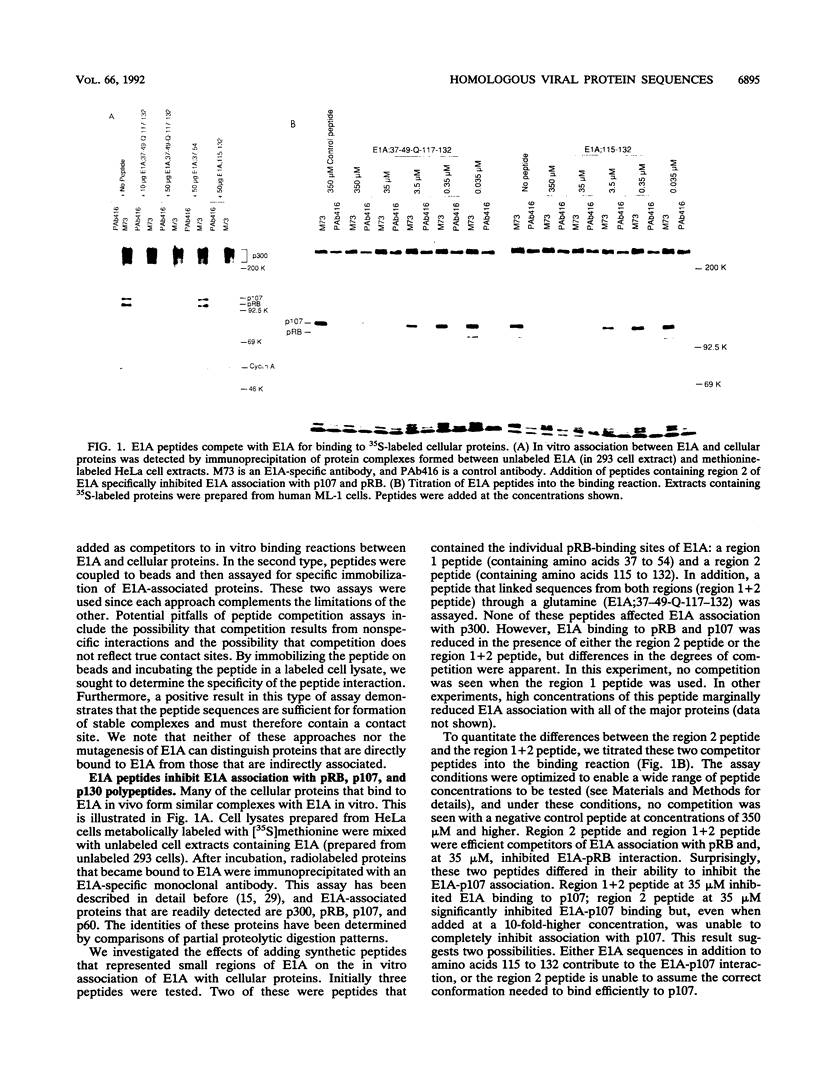
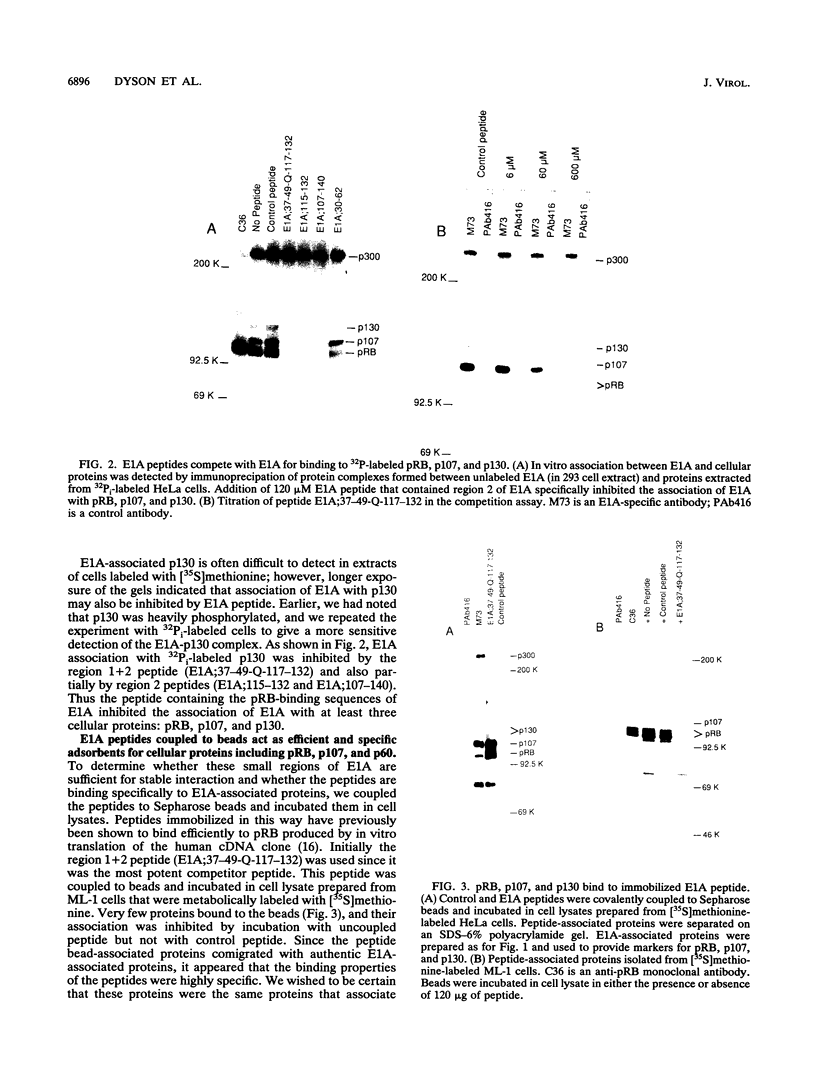
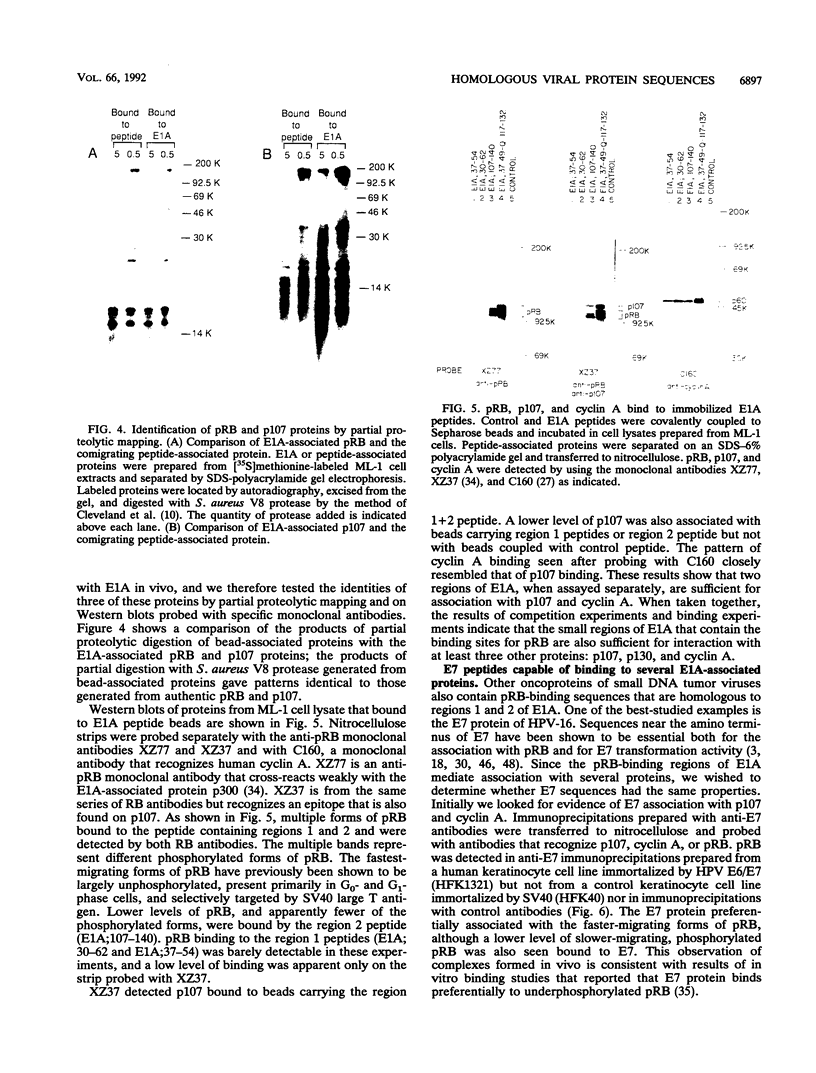

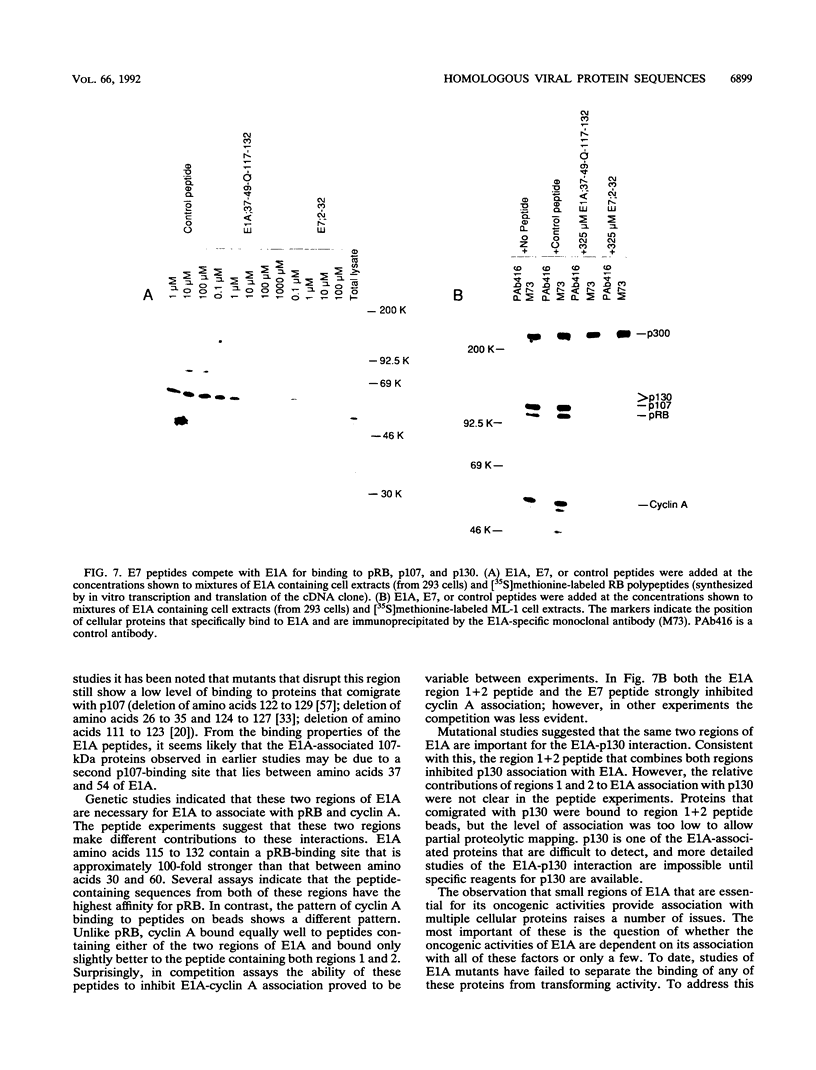

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandara L. R., Adamczewski J. P., Hunt T., La Thangue N. B. Cyclin A and the retinoblastoma gene product complex with a common transcription factor. Nature. 1991 Jul 18;352(6332):249–251. doi: 10.1038/352249a0. [DOI] [PubMed] [Google Scholar]
- Bandara L. R., La Thangue N. B. Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature. 1991 Jun 6;351(6326):494–497. doi: 10.1038/351494a0. [DOI] [PubMed] [Google Scholar]
- Barbosa M. S., Edmonds C., Fisher C., Schiller J. T., Lowy D. R., Vousden K. H. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and Sv40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 1990 Jan;9(1):153–160. doi: 10.1002/j.1460-2075.1990.tb08091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Cao L., Faha B., Dembski M., Tsai L. H., Harlow E., Dyson N. Independent binding of the retinoblastoma protein and p107 to the transcription factor E2F. Nature. 1992 Jan 9;355(6356):176–179. doi: 10.1038/355176a0. [DOI] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chellappan S., Kraus V. B., Kroger B., Munger K., Howley P. M., Phelps W. C., Nevins J. R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Devoto S. H., Mudryj M., Pines J., Hunter T., Nevins J. R. A cyclin A-protein kinase complex possesses sequence-specific DNA binding activity: p33cdk2 is a component of the E2F-cyclin A complex. Cell. 1992 Jan 10;68(1):167–176. doi: 10.1016/0092-8674(92)90215-x. [DOI] [PubMed] [Google Scholar]
- Dyson N., Bernards R., Friend S. H., Gooding L. R., Hassell J. A., Major E. O., Pipas J. M., Vandyke T., Harlow E. Large T antigens of many polyomaviruses are able to form complexes with the retinoblastoma protein. J Virol. 1990 Mar;64(3):1353–1356. doi: 10.1128/jvi.64.3.1353-1356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Buchkovich K., Whyte P., Harlow E. The cellular 107K protein that binds to adenovirus E1A also associates with the large T antigens of SV40 and JC virus. Cell. 1989 Jul 28;58(2):249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- Dyson N., Guida P., McCall C., Harlow E. Adenovirus E1A makes two distinct contacts with the retinoblastoma protein. J Virol. 1992 Jul;66(7):4606–4611. doi: 10.1128/jvi.66.7.4606-4611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Edmonds C., Vousden K. H. A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol. 1989 Jun;63(6):2650–2656. doi: 10.1128/jvi.63.6.2650-2656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C., Bayley S. T., Branton P. E. Binding of the Rb1 protein to E1A products is required for adenovirus transformation. Oncogene. 1989 Mar;4(3):383–388. [PubMed] [Google Scholar]
- Ewen M. E., Faha B., Harlow E., Livingston D. M. Interaction of p107 with cyclin A independent of complex formation with viral oncoproteins. Science. 1992 Jan 3;255(5040):85–87. doi: 10.1126/science.1532457. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Ludlow J. W., Marsilio E., DeCaprio J. A., Millikan R. C., Cheng S. H., Paucha E., Livingston D. M. An N-terminal transformation-governing sequence of SV40 large T antigen contributes to the binding of both p110Rb and a second cellular protein, p120. Cell. 1989 Jul 28;58(2):257–267. doi: 10.1016/0092-8674(89)90840-4. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Xing Y. G., Lawrence J. B., Livingston D. M. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. doi: 10.1016/0092-8674(91)90038-z. [DOI] [PubMed] [Google Scholar]
- Giordano A., Lee J. H., Scheppler J. A., Herrmann C., Harlow E., Deuschle U., Beach D., Franza B. R., Jr Cell cycle regulation of histone H1 kinase activity associated with the adenoviral protein E1A. Science. 1991 Sep 13;253(5025):1271–1275. doi: 10.1126/science.1653969. [DOI] [PubMed] [Google Scholar]
- Giordano A., McCall C., Whyte P., Franza B. R., Jr Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene. 1991 Mar;6(3):481–485. [PubMed] [Google Scholar]
- Giordano A., Whyte P., Harlow E., Franza B. R., Jr, Beach D., Draetta G. A 60 kd cdc2-associated polypeptide complexes with the E1A proteins in adenovirus-infected cells. Cell. 1989 Sep 8;58(5):981–990. doi: 10.1016/0092-8674(89)90949-5. [DOI] [PubMed] [Google Scholar]
- Harlow E., Whyte P., Franza B. R., Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck D. V., Yee C. L., Howley P. M., Münger K. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4442–4446. doi: 10.1073/pnas.89.10.4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert S. W., Blake M., Azizkhan J., Nevins J. R. Role of E2F transcription factor in E1A-mediated trans activation of cellular genes. J Virol. 1991 Jul;65(7):3547–3552. doi: 10.1128/jvi.65.7.3547-3552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert S. W., Chellappan S. P., Horowitz J. M., Nevins J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992 Feb;6(2):177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- Howe J. A., Mymryk J. S., Egan C., Branton P. E., Bayley S. T. Retinoblastoma growth suppressor and a 300-kDa protein appear to regulate cellular DNA synthesis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5883–5887. doi: 10.1073/pnas.87.15.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q. J., Bautista C., Edwards G. M., Defeo-Jones D., Jones R. E., Harlow E. Antibodies specific for the human retinoblastoma protein identify a family of related polypeptides. Mol Cell Biol. 1991 Nov;11(11):5792–5799. doi: 10.1128/mcb.11.11.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Matsushima Y., Sugimura T., Terada M. Purification and characterization of human papillomavirus type 16 E7 protein with preferential binding capacity to the underphosphorylated form of retinoblastoma gene product. J Virol. 1991 Sep;65(9):4966–4972. doi: 10.1128/jvi.65.9.4966-4972.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsma T. N., Howe J. A., Mymryk J. S., Evelegh C. M., Cunniff N. F., Bayley S. T. Sequences in E1A proteins of human adenovirus 5 required for cell transformation, repression of a transcriptional enhancer, and induction of proliferating cell nuclear antigen. Virology. 1989 Jul;171(1):120–130. doi: 10.1016/0042-6822(89)90518-7. [DOI] [PubMed] [Google Scholar]
- Jones R. E., Wegrzyn R. J., Patrick D. R., Balishin N. L., Vuocolo G. A., Riemen M. W., Defeo-Jones D., Garsky V. M., Heimbrook D. C., Oliff A. Identification of HPV-16 E7 peptides that are potent antagonists of E7 binding to the retinoblastoma suppressor protein. J Biol Chem. 1990 Aug 5;265(22):12782–12785. [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Ewen M. E., Livingston D. M. Definition of the minimal simian virus 40 large T antigen- and adenovirus E1A-binding domain in the retinoblastoma gene product. Mol Cell Biol. 1990 Jul;10(7):3761–3769. doi: 10.1128/mcb.10.7.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Pallas D. C., DeCaprio J. A., Kaye F. J., Livingston D. M. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991 Feb 8;64(3):521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larose A., St-Onge L., Bastin M. Mutations in polyomavirus large T affecting immortalization of primary rat embryo fibroblasts. Virology. 1990 May;176(1):98–105. doi: 10.1016/0042-6822(90)90234-i. [DOI] [PubMed] [Google Scholar]
- Mihara K., Cao X. R., Yen A., Chandler S., Driscoll B., Murphree A. L., T'Ang A., Fung Y. K. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science. 1989 Dec 8;246(4935):1300–1303. doi: 10.1126/science.2588006. [DOI] [PubMed] [Google Scholar]
- Mudryj M., Devoto S. H., Hiebert S. W., Hunter T., Pines J., Nevins J. R. Cell cycle regulation of the E2F transcription factor involves an interaction with cyclin A. Cell. 1991 Jun 28;65(7):1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 1989 Dec 20;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Yee C. L., Phelps W. C., Pietenpol J. A., Moses H. L., Howley P. M. Biochemical and biological differences between E7 oncoproteins of the high- and low-risk human papillomavirus types are determined by amino-terminal sequences. J Virol. 1991 Jul;65(7):3943–3948. doi: 10.1128/jvi.65.7.3943-3948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Münger K., Yee C. L., Barnes J. A., Howley P. M. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J Virol. 1992 Apr;66(4):2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol J. A., Stein R. W., Moran E., Yaciuk P., Schlegel R., Lyons R. M., Pittelkow M. R., Münger K., Howley P. M., Moses H. L. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990 Jun 1;61(5):777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Rustgi A. K., Dyson N., Bernards R. Amino-terminal domains of c-myc and N-myc proteins mediate binding to the retinoblastoma gene product. Nature. 1991 Aug 8;352(6335):541–544. doi: 10.1038/352541a0. [DOI] [PubMed] [Google Scholar]
- Scheffner M., Münger K., Byrne J. C., Howley P. M. The state of the p53 and retinoblastoma genes in human cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5523–5527. doi: 10.1073/pnas.88.13.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodkar S., Ewen M., DeCaprio J. A., Morgan J., Livingston D. M., Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992 Jan 10;68(1):157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Stein R. W., Corrigan M., Yaciuk P., Whelan J., Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990 Sep;64(9):4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Yee S. P., Branton P. E. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology. 1985 Nov;147(1):142–153. doi: 10.1016/0042-6822(85)90234-x. [DOI] [PubMed] [Google Scholar]