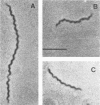
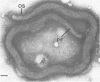
Abstract
Five strains of obligately anaerobic, pectin-fermenting spirochetes were isolated from the subgingival plaque of humans. The strains produced two extracellular enzymatic activities that functioned in pectin degradation. One of these enzymatic activities was pectin methylesterase (EC 3.1.1.11), and the other was pectate lyase (EC 4.2.2.2) of the endo type. The data indicate that the cumulative action of these two enzymatic activities brought about depolymerization of pectin in spirochete cultures. Pectin- or polygalacturonate-degrading hydrolases were not detected. A cell-associated lyase activity that catalyzed polygalacturonate breakdown was present in one of the spirochete strains. In addition to pectin, the isolates utilized polygalacturonic, glucuronic, or galacturonic acid as fermentable substrate but did not neutral sugars, amino acids, or other substrates tested. Although the oral spirochetes did not ferment hyaluronic acid, one of the strains grew in coculture with a hyaluronidase-producing Peptostreptococcus strain in a medium containing hyaluronic acid as fermentable substrate. Two of the isolates were identified as Treponema pectinovorum strains on the basis of their substrate utilization pattern, end products of fermentation, other phenotypic characteristics, and the guanine-plus-cytosine content of their DNA. Even though the pectinolytic isolates were specialized with respect to the fermentable substrates they utilized, they appeared to compete successfully with other microorganisms in their habitat.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Templeton B. The effect of environmental conditions on the motility of Escherichia coli. J Gen Microbiol. 1967 Feb;46(2):175–184. doi: 10.1099/00221287-46-2-175. [DOI] [PubMed] [Google Scholar]
- Armitage G. C., Dickinson W. R., Jenderseck R. S., Levine S. M., Chambers D. W. Relationship between the percentage of subgingival spirochetes and the severity of periodontal disease. J Periodontol. 1982 Sep;53(9):550–556. doi: 10.1902/jop.1982.53.9.550. [DOI] [PubMed] [Google Scholar]
- HASEGAWA S., NAGEL C. W. The characterization of an alpha, beta-unsaturated digalacturonic acid. J Biol Chem. 1962 Mar;237:619–621. [PubMed] [Google Scholar]
- Harwood C. S., Canale-Parola E. Adenosine 5'-triphosphate- yielding pathways of branched-chain amino acid fermentation by a marine spirochete. J Bacteriol. 1981 Oct;148(1):117–123. doi: 10.1128/jb.148.1.117-123.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K., Tateiri S., Iida H. Effects of pH of the medium on flagellation of Vibrio parahaemolyticus. Appl Environ Microbiol. 1979 Jun;37(6):1248–1249. doi: 10.1128/aem.37.6.1248-1249.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LISTGARTEN M. A., SOCRANSKY S. S. ELECTRON MICROSCOPY OF AXIAL FIBRILS, OUTER ENVELOPE, AND CELL DIVISION OF CERTAIN ORAL SPIROCHETES. J Bacteriol. 1964 Oct;88:1087–1103. doi: 10.1128/jb.88.4.1087-1103.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschine S. B., Canale-Parola E. Rifampin as a selective agent for isolation of oral spirochetes. J Clin Microbiol. 1980 Dec;12(6):792–795. doi: 10.1128/jcm.12.6.792-795.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listgarten M. A., Helldén L. Relative distribution of bacteria at clinically healthy and periodontally diseased sites in humans. J Clin Periodontol. 1978 May;5(2):115–132. doi: 10.1111/j.1600-051x.1978.tb01913.x. [DOI] [PubMed] [Google Scholar]
- McCOMB E. A., McCREADY R. M. Use of the hydroxamic acid reaction for determining pectinesterase activity. Stain Technol. 1958 May;33(3):129–131. doi: 10.3109/10520295809111836. [DOI] [PubMed] [Google Scholar]
- McGroarty E. J., Koffler H., Smith R. W. Regulation of flagellar morphogenesis by temperature: involvement of the bacterial cell surface in the synthesis of flagellin and the flagellum. J Bacteriol. 1973 Jan;113(1):295–303. doi: 10.1128/jb.113.1.295-303.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran F., Nasuno S., Starr M. P. Extracellular and intracellular polygllacturonic acid trans-eliminases of Erwinia carotovora. Arch Biochem Biophys. 1968 Feb;123(2):298–306. doi: 10.1016/0003-9861(68)90138-0. [DOI] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonase of Erwinia carotovora. J Biol Chem. 1966 Nov 25;241(22):5298–5306. [PubMed] [Google Scholar]
- Paster B. J., Canale-Parola E. Physiological diversity of rumen spirochetes. Appl Environ Microbiol. 1982 Mar;43(3):686–693. doi: 10.1128/aem.43.3.686-693.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSAN B., WILLIAMS N. B. HYALURONIDASE PRODUCTION BY ORAL ENTEROCOCCI. Arch Oral Biol. 1964 May-Jun;9:291–298. doi: 10.1016/0003-9969(64)90061-5. [DOI] [PubMed] [Google Scholar]
- Rexová-Benková L., Markovic O. Pectic enzymes. Adv Carbohydr Chem Biochem. 1976;33:323–385. doi: 10.1016/s0065-2318(08)60285-1. [DOI] [PubMed] [Google Scholar]
- SCHULTZ-HAUDT S. D., SCHERP H. W. The production of chondrosulfatase by microorganisms isolated from human gingival crevices. J Dent Res. 1956 Apr;35(2):299–307. doi: 10.1177/00220345560350022301. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Listgarten M., Hubersak C., Cotmore J., Clark A. Morphological and biochemical differentiation of three types of small oral spirochetes. J Bacteriol. 1969 Jun;98(3):878–882. doi: 10.1128/jb.98.3.878-882.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton T. B., Canale-Parola E. Enumeration and selective isolation of rumen spirochetes. Appl Environ Microbiol. 1979 Nov;38(5):965–973. doi: 10.1128/aem.38.5.965-973.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowicz M., Ziołecki A. Pectinolytic enzymes of large rumen treponemes. Appl Environ Microbiol. 1979 Jan;37(1):136–142. doi: 10.1128/aem.37.1.136-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziołecki A., Wojciechowicz M. Small pectinolytic spirochetes from the rumen. Appl Environ Microbiol. 1980 Apr;39(4):919–922. doi: 10.1128/aem.39.4.919-922.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]